in part a, you determined that 98.0 g of h2o is equal to 5.44 mol of h2o . you then multiplied the number of moles by the heat of fusion to find the energy needed for melting. part c is similar to part a, except that you will use the heat of vaporization instead of the heat of fusion to find the energy needed for boiling.
Answers
The energy required to vaporize 98.0 g of H2O is 221 kJ. This process requires a lot more energy than melting, as the heat of vaporization is much greater than the heat of fusion.
In part a, we found the energy required to melt ice by using the heat of fusion. Now, in part c, we need to find the energy required to vaporize water. To do this, we need to use the heat of vaporization, which is the amount of energy required to convert a substance from a liquid to a gas. The heat of vaporization of water is 40.7 kJ/mol.
We already know that 98.0 g of H2O is equal to 5.44 mol of H2O (from part a). Now, we can multiply the number of moles by the heat of vaporization to find the energy required for boiling:
Energy = 5.44 mol x 40.7 kJ/mol = 221 kJ
So, the energy required to vaporize 98.0 g of H2O is 221 kJ. This process requires a lot more energy than melting, as the heat of vaporization is much greater than the heat of fusion. It takes a significant amount of energy to break the bonds between liquid molecules and allow them to escape into the gas phase.
To know more about heat of fusion visit: https://brainly.com/question/32292861
#SPJ11
Related Questions
The levels of organizations in multicellular organisms are shown below, but they are not in the correct order. Arrange the levels of organization in order from simplest to most complex.
Answers
The question is incomplete, the complete question is;
The levels of organizations in multicellular organisms are shown below, but they are not in the correct order. Arrange the levels of organization in order from simplest to most complex. tissues organs whole organisms organ systems
Answer:
tissues --> organs --> organ system --> whole organisms
Explanation:
The levels of organizations in multicellular organisms refers to the increasing complexity of the components of the organism.
A tissue is an aggregation of cells hence it is the lowest level of organization here.
Organs are an aggregation of tissues, this is the next higher level of organization.
Organ systems are an aggregation of organs, this is the next higher level of organization.
Whole organisms are an aggregation of organ systems. This is the highest level of organization.
what do you think will be the effect of the different temperature, pH and salinity on the speed of Alka-Seltzer dissolving
Answers
Temperature and pH can potentially affect the speed of Alka-Seltzer dissolving, with higher temperatures and lower pH levels likely increasing the dissolution rate. Salinity, on the other hand, generally has minimal impact.
The speed of Alka-Seltzer dissolving can be affected by temperature, pH, and salinity. Here's a brief explanation of their potential effects:
1. Temperature: Increasing the temperature typically enhances the rate of dissolution. Higher temperatures provide more thermal energy, leading to faster molecular movement and collisions, thereby increasing the dissolution rate of Alka-Seltzer.
2. pH: Alka-Seltzer contains citric acid and sodium bicarbonate, which react to produce carbon dioxide gas. pH can influence the rate of this chemical reaction. Lower pH levels (more acidic conditions) may accelerate the reaction, resulting in faster dissolution. However, extreme pH levels, particularly highly acidic or highly alkaline conditions, might have an adverse effect on the dissolution process.
3. Salinity: Salinity refers to the salt content in the surrounding solution. Salinity generally does not have a significant impact on the dissolution of Alka-Seltzer tablets. As long as the water used for dissolving Alka-Seltzer is not excessively saline, the presence of salts should not noticeably affect the dissolution rate.
It's important to note that these factors may interact with each other, and the specific composition and formulation of Alka-Seltzer can also influence the dissolution behavior. Experimental testing under different conditions would provide more accurate and detailed insights into how temperature, pH, and salinity affect the speed of Alka-Seltzer dissolving.
To know more about Alka-Seltzer refer here
https://brainly.com/question/14530751#
#SPJ11
The atomic number of oxygen is 8. What is the mass number of a oxygen atom with 7 neutrons?.
Answers
The atomic number of oxygen is 8. The mass number of an oxygen atom with 7 neutrons is 15.
What is atomic mass?Atomic mass is the average mass of the atom. It is measured in atomic mass units, that is Dalton. The mass number is calculated by the number of neutrons and atomic number.
The atomic number is the number of protons present in the atoms. The atomic number of oxygen is 8
The mass number can be calculated by adding those units
atomic number + number of neutrons
Mass number = 8 + 7 = 15
Thus, the mass number of an oxygen atom with 7 neutrons is 15.
To learn more about atomic mass, refer to the link:
https://brainly.com/question/8101390
#SPJ4
When h2so4 is neutralized by naoh in aqueous solution, the net ionic equation is ________.
Answers
When H₂SO₄ is neutralized by NaOH in an aqueous solution, the net ionic equation is:
2H⁺(aq) + 2OH⁻(aq) → 2H₂O(l)
A chemical equation known as an ionic equation expresses the electrolytes in an aqueous solution as dissociated ions. For the reaction between \(H_{2}SO_{4}\) , NaOH that neutralizes it, we must write the net ionic equation.
The reaction's equilibrium chemical equation is:
\(Na_{2} SO_{4}\)(aq) + \(2H_{2}O\) = \(H_{2}SO_{4}\) (aq) + \(2NaOH\)(aq) (l)
We shall now draft the ionic equation.
The reaction's ionic equation is
\(2 Na ^{+} (aq) + SO_{4} ^{2-} (aq) + 2H_{2}O = 2H^{+} (aq) + SO_{4} ^{2-} (aq) + 2 Na ^{+} (aq)+ 2OH(aq) (l)\)
The net ionic equation is then obtained by canceling out the spectator ions Na+ and SO42.
The neutralization reaction's net ionic equation is:
2H⁺(aq), 2OH⁻(aq), and 2H₂O (l)
In light of this, the net ionic equation for the neutralization of H2SO4 and NaOH is:
2H⁺(aq) + 2OH⁻(aq) → 2H₂O(l)
To know more about ionic equation refer to: https://brainly.com/question/15466794
#SPJ4
Curious Carl and his lab partner were handed a 2 liter sealed flask containing two gases, neon and argon. The partial of each gas
was 101.3 kPa. Carl and his partner then forced the gases into a smaller, 1 liter container.
What is the new partial pressure of each gas and the total pressure exerted on the container?
es
A)
50.65 kPa; 101.3 kPa
B)
101.3 kPa; 202.6 kPa
C)
202.6 kPa; 405.2 kPa
D)
202.6 kPa; 202.6 kPa
Answers
C) 202.6 kPa; 405.2 kPa
When Carl and his lab partner force the gases into a smaller, 1 liter container, the total pressure exerted on the container will increase based on Boyle's Law. Boyle's Law states that the pressure and volume of a gas are inversely proportional at constant temperature.
Since the original total pressure of each gas is 101.3 kPa, the new total pressure in the smaller container will be double that amount, resulting in 202.6 kPa. This eliminates options A and B.
The partial pressure of each gas will remain the same as before, even when the volume changes. This is because the gases are confined to the same volume ratio within the new container. Therefore, the new partial pressure of each gas will still be 101.3 kPa.
So, the correct answer is:
C) 202.6 kPa; 405.2 kPa
To know more about partial pressure click this link -
brainly.com/question/30114830
#SPJ11
ANSWER FAST
What is the relationship between Ka and Kb with Kw?
(a) The sum of Ka and Kb equals the auto-dissociation constant for water.
(b) The product of Ka and Kb equals the auto-dissociation constant for water.
(c) The quotient of Ka and Kb equals the auto-dissociation constant for water.
(d)The difference of Ka and Kb equals the auto-dissociation constant for water.
Answers
What is the molar mass of Ca(NO3)2?
102. 2 g/mol
134. 1 g/mol
164. 1 g/mol
204. 2 g/mol
Answers
Answer:
The answer is 164.1 g/mol
Explanation:
The semiconductor gallium arsenide, GaAsGaAs, is used in highspeed integrated circuits, light-emitting diodes, and solar cells. Its density is 5.32 g/cm3g/cm3. It can be made by reacting trimethylgallium, Ga(CH3)3Ga(CH3)3, with arsine gas, AsH3AsH3. The other product of the reaction is methane, CH4CH4. Part A Part complete If you reacted 450 gg of trimethylgallium with 300 gg of arsine, what mass of GaAsGaAs could you make
Answers
To determine the mass of GaAsGaAs that can be made from 450 g of trimethylgallium and 300 g of arsine, we need to know the stoichiometry of the reaction. The balanced chemical equation for the reaction of trimethylgallium and arsine to form GaAsGaAs is:
Ga(CH3)3 + AsH3 → GaAs + 3CH4
From this equation, we can see that for every mole of Ga(CH3)3 reacted with one mole of AsH3, we will get one mole of GaAs and three moles of CH4.
Since we have 450 g of trimethylgallium and we want to make 1 mole of GaAsGaAs, we can calculate the mass of trimethylgallium needed as follows:
Mass of GaAsGaAs = 1 mole
Mass of GaAs = 1 mole / molar mass of GaAs = 1 / 65.39 g/mol = 0.0152 g/mol
Mass of trimethylgallium = 450 g / molar mass of Ga(CH3)3 = 450 / 99.75 g/mol = 4.5 mol
Since we want to make 1 mole of GaAsGaAs, we can calculate the mass of arsine needed as follows:
Mass of arsine = Mass of GaAsGaAs / Molar mass of GaAsGaAs = 1 mole / 0.665 g/mol = 1.46 g
Therefore, we can make 1 mole of GaAsGaAs from 450 g of trimethylgallium and 300 g of arsine, and the total mass of the product will be 450 g + 300 g = 750 g.
Learn more about GaAsGaAs visit: brainly.com/question/11287988
#SPJ11
NEED BY HAND SOLUTION OF THIS PROBLEM DO NOT SOLVE ON EXCEL, SHOW STEP-BY-STEP SOLUTION FOR LIKE
The city of Carlsbad in California is considering building a $300 million water-desalination plant. The facility would be the largest in the Western Hemisphere, producing 50 million gallons of drinking water a day – enough to supply about 100,000 homes. This plant, which uses improved membranes and pumping system to bring down the energy cost to $0.90 in electricity to produce 10,000 gallons of water. Suppose the desalination plant is fully operational and you expect to operate it continuously for A0 years, with an estimated salvage value of $20 million. The operating and maintenance costs (excluding energy cost) amount to $15 million the first year and will increase at a rate of 3% per year. If the city plans to sells the water for about $925 per acre-foot (an acre-foot is 325,851 gallons – enough water for four people per year.), can it recover the capital cost and other operating costs? Assume the city’s interest rate for this water project is known to be 6%.
Answers
The initial investment is $300 million, and the project is planned to operate for A0 years with an estimated salvage value of $20 million.
To calculate whether the city can recover the capital cost and other operating costs for the water-desalination plant, you can follow these step-by-step calculations:
Step 1: Calculate the annual revenue from selling water:
First, convert the water production from gallons to acre-feet:
Water production per day = 50 million gallons
Water production per year = 50 million gallons * 365 days = 18.25 billion gallons
Water production in acre-feet = 18.25 billion gallons / 325,851 gallons per acre-foot
Calculate the annual revenue from selling water:
Annual revenue = Water production in acre-feet * Price per acre-foot
Step 2: Calculate the annual operating and maintenance costs (excluding energy cost):
Operating and maintenance costs in the first year = $15 million
Calculate the annual operating and maintenance costs for subsequent years:
Operating and maintenance costs in year n = Operating and maintenance costs in year n-1 * (1 + Growth rate)
Step 3: Calculate the annual energy cost:
Energy cost per 10,000 gallons = $0.90
Calculate the annual energy cost:
Annual energy cost = Water production in gallons / 10,000 gallons * Energy cost per 10,000 gallons
Step 4: Calculate the annual cash flow:
Annual cash flow = Annual revenue - Annual operating and maintenance costs - Annual energy cost
Step 5: Calculate the present value of cash flows:
Use the present value formula to calculate the present value of cash flows over the A0 years:
PV = Annual cash flow / (1 + Interest rate)^Year
Step 6: Calculate the net present value (NPV):
NPV = PV - Initial investment
Step 7: Determine if the project is financially feasible:
If the NPV is greater than or equal to zero, the project is financially feasible and can recover the capital cost and other operating costs. If the NPV is negative, the project may not be able to recover the costs.
To know more about cash flows
https://brainly.com/question/27994727
#SPJ11
Question 1 of 10
Which two terms apply to oceanic crust rather than continental crust?
A. Thicker
O B. Lighter in color
0 C. Denser
I D. Younger in age
Answers
The two terms apply to oceanic crust rather than continental crust C. Denser and D. Younger in age
What is the oceanic crust made up of?
Oceanic Crust Oceanic crust, extending 5-10 kilometers (3-6 kilometers) beneath the ocean floor, is mostly composed of different types of basalts. Geologists often refer to the rocks of the oceanic crust as “sima.” Sima stands for silicate and magnesium, the most abundant minerals in oceanic crust.
What is an example of oceanic crust?Oceanic crust is thin (6 km thick) and dense (about 3.3 g/cm), consisting of basalt, gabbro, and peridotite. They include oceanic sediments (e.g. radiolarites, turbidites) and oceanic crust (e.g. basalt, pillow lava).
Learn more about Oceanic crust here https://brainly.com/question/26053779
#SPJ2
what reaction is used for anabolism? group of answer choices hydrolysis catabolic dehydration hydration
Answers
The reaction is used for the anabolism is the dehydration. that means the loss of the water molecules.
The water is the universal solvent. water is the chemically reactive compound and the number of the biochemical reactions takes place. The hydrolysis is the reaction in which use water molecule break the bonds in between the large compounds. the hydrolysis reaction is used in the catabolism. such as the depolymerization of the protein molecules.
In the condensation reaction , the two molecules joined to form the large molecule. this reaction will leads to the water molecule loss. this is called dehydration reaction. the dehydration reaction used in the anabolism reaction or anabolic reaction.
To learn more about anabolic here
https://brainly.com/question/14932822
#SPJ4
which reaction is not a reduction–oxidation reaction?
a. CH4 + 2O2 → CO2 + 2H2O
b. Fe2O3 + 3C → 2Fe + 3CO
c. 3CuO + 2NH3 → 3Cu + N2 + 3H2O
d. Ca(OH)2 + 2CH3COOH → Ca(CH3COO)2 + 2H2O
Answers
The reaction is the reduction – oxidation reaction is the :
Ca(OH)₂ + 2CH₃COOH → Ca(CH₃COO)₂ + 2H₂O. The correct option is d.
The gain of the electron is the reduction. In the reduction the oxidation number will increases. The loss of the electron during the reaction is called the oxidation and the decrease in the oxidation number is the oxidation. When both occur in the reaction the reaction is said to be the redox reaction or the reduction – oxidation .
The reaction is as:
Ca(OH)₂ + 2CH₃COOH → Ca(CH₃COO)₂ + 2H₂O
This is not the redox reaction as the oxidation states of the multiple atoms is not change during the reaction.
To learn more about oxidation - reduction here
https://brainly.com/question/19528268
#SPJ4
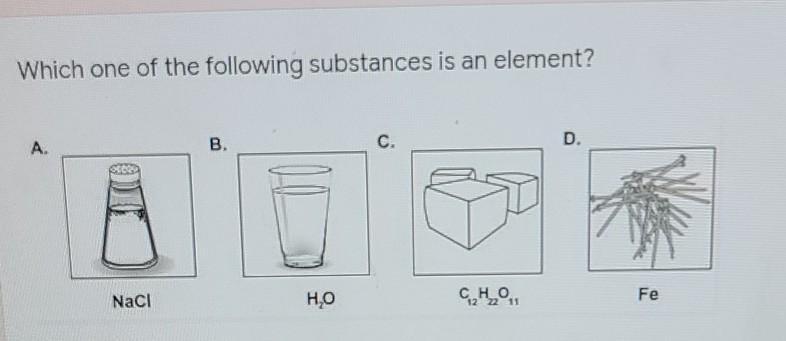
Which one of the following substances is an element?
Answers
Answer: Fe
Explanation: Fe (Iron) is the only element since it involves only 1 atom. The other options are compounds, since there are more than 2 atoms bonded together.
What are the (3) most commonly used chemical sanitizers and their PPM?
Answers
bleach) containing 50 to 100 micrograms (ppm) of chlorine. The most common sanitizers on the market contain chlorine. the quaternary ammonium (QUAT, QAC) Maintaining concentration: as instructed by the manufacturer. Iodine. Concentration ranges from 12.5 to 25 ppm.
What disinfectants spring to mind?The three main chemical substances that are used as sanitizers in the food service industry are iodine sanitizers, chlorine-based cleansers, and cleaners. The most used molecular sanitizing agent is chlorine since it is both very effective and inexpensive.
Is rubbing alcohol a sanitizer?It may successfully eradicate a variety of pathogens, including bacteria, hepatitis, and fungi, due to its effectiveness as a germicide. Rubing alcohol can be used as a household cleaner, but it is widely employed in medical settings to clean hands.
To know more about Sanitizers visit:
https://brainly.com/question/1570533
#SPJ4
a solution contains 1.569 mg of coso4 (155.0 g/mol) per milliliter. calculate a. the volume of 0.007840 m edta needed to titrate a 25.00 ml aliquot of this solution. b. the volume of 0.009275 m zn2 needed to titrate the excess reagent after addition of 50.00 ml of 0.007840 m edta to a 25 ml aliquot of this solution
Answers
Volume of EDTA needed is \(32.28ml\) and Volume of \(Zn^{+2}\) needed to titrate the excess reagent is \(14.9796ml\).
Titration-a procedure or method for calculating the concentration of a dissolved substance using the least amount of reagent at a certain concentration needed to get a specific result when combined with a known volume of the test solution.
concentration refers to the amount of a substance in a defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution.
Concentration of \(Co^{+2}\)=\((\frac{1.569\times10^{-3} }{155} )\times(\frac{1000}{1} )=0.0101226M\)
\(M_{1}V_{1} =M_{2}V_{2}\)\(V_{1}=\frac{0.0101226M\times25ml}{0.007840M}\)
\(V_{1}=32.2786ml\)
Volume of EDTA needed is\(32.28ml\)
millimoles of EDTA taken = \(0.007840M\times 50.00ml = 0.392mmol\)millimoles of \(CO^{+2}\) in the sample is \(0.0101226M\times25.00ml = 0.25306mmol\)
millimoles of EDTA(excess)=\(0.392 - 0.25306 = 0.138935mmol\)= millimoles of \(Zn^{+2}\) reacted
Volume of \(Zn^{+2}\) needed = \(\frac{0.138835mmol}{0.009275M } = 14.9796\:ml\)
Volume of \(Zn^{+2}\) needed to titrate the excess reagent\(=14.9796ml\)
Learn more about the Titration
brainly.com/question/29509561
#SPJ4
a teacher divides her class into groups and assigns each group a task of measuring the mass of the same object three times.the teacher already knows that the mass of the object is 25g based on the results each group records,which group makes the most precise measurement of the object
Answers
Answer:
This question lacks options, the options are:
A. Group C: 32.1 g, 35.0 g, 25.0 g
B. Group B: 25.5 g, 25.0 g, 24.8 g
C. Group A: 28.5g, 28.4 g, 28.5 g
D. Group D: 20.0 g, 25.0 g, 30.09
The answer is C. GROUP A
Explanation:
Precision in measurements refers to the degree of closeness between the repeated or measured values irrespective of how close they are to the true or accepted value, which is 25g in this case.
The precision of a measurement can be determined by simply finding the range (highest - lowest) of the measurements. The lowest range represents the most precise. The measurements for each group are:
Group C: 32.1 g, 35.0 g, 25.0 g
Range = 35.0 - 25.0 = 10g
Group B: 25.5 g, 25.0 g, 24.8 g
Range = 25.5 - 24.8 = 0.7g
Group A: 28.5g, 28.4 g, 28.5 g
Range: 28.5 - 28.4 = 0.1g
Group D: 20.0 g, 25.0 g, 30.09
Range = 30.09 - 25.0= 5.09g
Based on the ranges of the measurements in each group, one would notice that Group A has the lowest range (0.1g), hence, GROUP A is the most precise.
Answer: group A
Explanation: I got the answer right on my quiz.
a flask has a mass of 78.23 g when empty and 593.63 g when filled with water. when the same flask is filled with concentrated sulfuric acid, h2so4, its mass is 1026.57 g. what is the density of concentrated sulfuric acid in g/cm3? (assume water has a density of 1.00 g/cm3 at the temperature of the measurement.)
Answers
the density of concentrated sulfuric acid is 1.84 g/mL when a flask has a mass of 78.23 g when empty and 593.63 g when filled with water and later with sulfuric acid whose mass is 1026.57 g.
Given mass of flask mf = 78.23 g
Mass of flask when filled with water mfw = 593.63 g
Mass of water mw = mfw - mf = 593.63 - 78.23 = 515.4g
Volume of water Vw = Volume of acid Vc = 515.4mL
Mass of flask when filled with acid mfa = 1026.57 g
Mass of acid ma = mfa-mf = 1026.57 - 78.23 = 948.34g
Density = mass(ma)/volume of acid (Va)
= 948.34/515.4 = 1.84 g/mL
To learn more about sulfuric acid click here https://brainly.com/question/28513840
#SPJ4
Crystallization is a process of _________
Answers
Crystallization is a process of separation of the solid substances in an object located in the solution as crystal particles.
What is the chemical process of Crystallization?The chemical process of Crystallization refers to a procedure in which we apply different methods in a solution in order to separate crystals, which are solids compounds that may be considered solutes in such media.
Therefore, with this data, we can see that the chemical process of crystallization separates the solutes in a given solution as solid crystal particles that can be used in other applications.
Learn more about the chemical process of crystallization here:
https://brainly.com/question/5520192
#SPJ1
How do I calculate the spin and orbit of lithium and
deuteron?
Answers
The spin and orbit of lithium and deuteron are:
Lithium: Spin can be either 3/2 or 1/2 and orbit is 1s² 2s¹.
Deuteron: Spin can be either 1 or 0. The concept of electron orbitals does not apply to the deuteron as a nucleus.
To calculate the spin and orbit of lithium and deuteron, we need to understand the quantum mechanical properties of these particles.
Lithium: Lithium is an atom with three electrons. Each electron has a spin of 1/2, as electrons are fermions and follow the Pauli exclusion principle. The total spin of an atom is determined by the combination of the individual electron spins. In the case of lithium, the three electron spins can combine in different ways.
If all three electrons have their spins aligned in the same direction (spin-up), the total spin is 3/2.If two electrons have their spins aligned in the same direction and one is opposite (spin-up, spin-up, spin-down), the total spin is 1/2.The orbit of lithium refers to the arrangement of its electrons around the nucleus. The specific orbitals and their arrangement depend on the electron configuration of lithium, which is 1s² 2s¹. This means that there are two electrons in the 1s orbital and one electron in the 2s orbital.
Deuteron: Deuteron is a nucleus of an isotope of hydrogen, known as heavy hydrogen or hydrogen-2. It consists of one proton and one neutron. Both the proton and neutron are fermions and have a spin of 1/2.
The total spin of the deuteron is determined by the combination of the proton and neutron spins. Since there is only one proton and one neutron, their spins can combine in two ways:
If the spins of the proton and neutron are aligned in the same direction (spin-up), the total spin is 1.If the spins of the proton and neutron are aligned in opposite directions (spin-up, spin-down), the total spin is 0.The orbit of the deuteron, in the context of the deuteron as a nucleus, refers to the motion of the nucleus in an atomic or molecular system. It is important to note that the deuteron, being a nucleus, does not possess electron orbitals like atoms.
To know more about deuteron here
https://brainly.com/question/32899336
#SPJ4
Classify each of the following elements as
a metal, a nonmetal, or a metalloid:
boron, carbon, aluminum, and silicon.
Answers
The non-metals are boron and carbon. The metal is aluminum and the metalloid is silicon.
Explanation:
non-metal = boron, carbon
metal = aluminum
metalloid = silicon
Non-metal = boron, carbon
Metal = aluminum
Metalloid = silicon
What is metal?A solid material which is lustrous, hard, shiny, malleable, and sonorous with good electrical and thermal conductivity.
Metal is an element which is typically hard, shiny, fusible, malleable, and ductile, with good electrical and thermal conductivity.
A nonmetal is an element that does not have the properties of a metal.
A metalloid is an element having intermediate properties of both metals and nonmetals.
Hence,
Non-metal = boron, carbon
Metal = aluminum
Metalloid = silicon
Learn more about metals here:
https://brainly.com/question/16759172
#SPJ2
It takes a force of 3000 N to accelerate an empty 1000 kg car at 3 m/s2. If a 160
kg wrestler is inside the car, how much force will be needed to produce the same
acceleration? *
Answers
Answer:
the force needed to produce the same acceleration is 480kg
About how long does it take the Sun to rotate once about its axis?
A. 30 seconds
B. 10 minutes
C. 25,000 Earth years
D. 25 Earth days
Answers
Answer:
its actually supposed to be 27 days
Which variable is unknown until the experiment is performed?
Answers
The variable that is unknown until the experiment is performed is the dependent variable.
In a scientific experiment, variables are classified into two main categories: independent variables and dependent variables. The independent variable is the variable that is intentionally manipulated or changed by the experimenter. It is under the control of the experimenter and is deliberately altered to observe its effect on the dependent variable.
On the other hand, the dependent variable is the variable that is measured or observed as the outcome or response in the experiment. It is the variable that is expected to change in response to the manipulation of the independent variable. The value or behavior of the dependent variable depends on the value or behavior of the independent variable.
Typically, before conducting an experiment, researchers have a hypothesis or an expectation about how the independent variable will affect the dependent variable. However, the actual outcome or result of the experiment, which is observed through the measurement of the dependent variable, remains unknown until the experiment is performed.
The purpose of conducting the experiment is to gather empirical data and observe the changes in the dependent variable to analyze the relationship between the independent and dependent variables.
For more such questions on dependent variable visit:
https://brainly.com/question/28433016
#SPJ8
Some analytes must be derivatized to increase their column retention or detectability. Derivatization means Group of answer choices altering the chemical structure of the analyte to increase detection and specificity. adding fluorescent labels or combining the analyte with chiral reagents or other chemicals to increase detectability. removing dissolved gases in the solvent to produce a clear chromatogram. using multiple detectors to assist in identification.
Answers
Derivatization means adding fluorescent labels or combining the analyte with chiral reagents or other chemicals to increase detectability.
Some analytes must be derivatized to increase their column retention or detectability.
Retention time can be referred to as the amount of time a solute spends in the stationary and mobile phases of a column.
Detectability is the ability of an analyte to get detected in the mobile phase of chromatography.
The refractometer, fluorescence detector, and UV detector are the three most popular liquid chromatography detectors. These detectors increase the detectability.
For derivatization, the fluorescence detector are used.
Learn more about derivatization
https://brainly.com/question/17609332
#SPJ4
what is the iupac name of OHC(CH2)3CH(CH3)CO2H
Answers
The IUPAC name of OHC(CH2)3CH(CH3)CO2H is 4-hydroxy-4-methylpentanoic acid.
What is the iupac name of OHC(CH2)3CH(CH3)CO2H?
To determine the IUPAC name of the organic compound, we will follow the steps below:
The longest chain of carbon atoms in the molecule contains 6 carbons. So, the base name is pentanoic acid.The carboxylic acid group (-CO2H) is attached to carbon-1 of the pentanoic acid chain. So, the full name is pentanoic acid.There is a hydroxyl group (-OH) attached to carbon-4 of the pentanoic acid chain. So, the prefix hydroxy is added and the name becomes 4-hydroxypentanoic acid.There is a methyl group (-CH3) attached to carbon-4 of the pentanoic acid chain. So, the prefix 4-methyl is added and the name becomes 4-methyl-4-hydroxypentanoic acid.Finally, the positions of the hydroxyl and methyl groups are indicated by the lowest possible numbers and the name becomes 4-hydroxy-4-methylpentanoic acid.Learn more about IUPAC name here: https://brainly.com/question/28872356
#SPJ1
How does a winnowing basket work
Answers
Answer:
The farmer places the wheat in the winnowing basket after flailing the grain. The threshing barn doors are opened on windy days. The farmer throws the grain into the air using the winnowing basket, where the wind removes the chaff.
Explanation:
what are the products in the chemical reaction of vinegar and baking soda to form sodium acetate water and carbon dioxide
Answers
Salt (sodium lactate), water, or carbon dioxide gases are the products of a reaction that happens when hydrogen peroxide (sodium bicarbonate) or vinegar (acetic anhydride) are mixed.
A chemical reaction is what?One or more chemicals, also referred to as reactants, are transformed into one or more other substances, often referred to as products, in a chemical reaction. Substances are constructed up of chemical components or chemical elements. A chemical reaction occurs once a or more chemicals change into one or several other substances. An illustration of this is when iron and gas mix to produce rust. Vinegar and soda soda together result in the production of water, carbon dioxide, and sodium acetate.
To know more about Chemical reaction visit:
brainly.com/question/29039149
#SPJ1
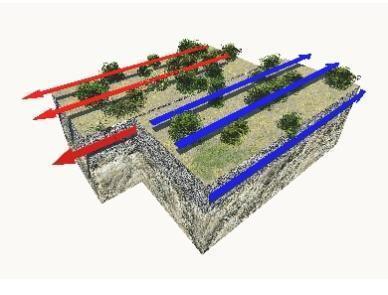
Study the image.
Which phrase describes this plate boundary?
does not occur in oceans
may form rift valleys
is a type of convergent boundary
is a region where earthquakes occur
Answers
The phrase that describes the plate boundary shown is D. is a region where earthquakes occur .
What is the plate boundary shown ?A transform boundary is the name given to this tectonic plate boundary. Two or more plates slip past one another in such a border. This shift is typically accompanied by some highly active earthquakes.
At a transform plate border, the grinding motion between the plates causes shallow earthquakes, significant lateral rock displacement, and a wide zone of crustal deformation. Essentially While no new crust is produced, subducted, or formed, and no volcanoes are formed, the fault produces earthquakes.
Find out more on plate boundaries at https://brainly.com/question/22005072
#SPJ1
a hydrochloric acid solution HCI has a concentration of 12.1 M. A 41.2 mL sample is used to make a more dilute solution. If the new solution has a concentration of 0.5 M determine the volume of the solution
Answers
If the new solution has a concentration of 0.5 M, then the volume of the solution is 997mL.
How do we calculate the required volume?Required volume of the new solution will be calculated by using the below equation as:
M₁V₁ = M₂V₂, where
M₁ & V₁ are the molarity and volume of initial HCl solution.
M₂ & V₂ are the molarity and volume of final HCl solution.
On putting values from the question, to the above question we get:
V₂ = (12.1)(41.2) / (0.5) = 997 mL
Hence resultant volume of the final solution is 997 mL.
To know more about concentration & volume, visit the below link:
https://brainly.com/question/7208546
#SPJ1
Seawater containing 3.50 wt% salt passes through a series of 10 evaporators. Roughly equal quantities of water are vaporized in each of the 10 units and then condensed and combined to obtain a product stream of fresh water. The brine leaving each evaporator but the tenth is fed to the next evaporator. The brine leaving the tenth evaporator contains 5.00 wt% salt.
(a) Draw a flowchart of the process showing the first, fourth, and tenth evaporators. Label all the streams entering and leaving these three evaporators.
(b) Write in order the set of equations you would solve to determine the fractional yield of fresh water from the process (kg H2O recovered/kg H2O in process feed) and the weight percent of salt in the solution leaving the fourth evaporator. Each equation you write should contain no more than one previously undetermined variable. In each equation, circle the variable for which you would solve. Do not do the calculations.
(c) Solve the equations derived in Part (b) for the two specified quantities.
Answers
The fractional yield of fresh water from the process is determined by solving a set of equations. The weight percent of salt in the solution leaving the fourth evaporator can also be determined using these equations.
(a) Flowchart of the process:
In the first evaporator, seawater containing 3.50 wt% salt enters and produces vaporized water with the same salt concentration. The vaporized water is condensed, and the resulting brine, still containing 3.50 wt% salt, is fed to the second evaporator. This process continues until the tenth evaporator, where the brine leaving has a salt concentration of 5.00 wt%. The product stream of fresh water is obtained by condensing and combining the vaporized water from each evaporator.
(b) Set of equations:
To determine the fractional yield of fresh water from the process and the weight percent of salt in the solution leaving the fourth evaporator, the following equations can be solved:
Equation 1: x1 = (1 - 0.035) [Variable to solve: x1]
Equation 2: x2 = (1 - 0.035) * x1 [Variable to solve: x2]
Equation 3: x3 = (1 - 0.035) * x2 [Variable to solve: x3]
Equation 4: x4 = (1 - 0.035) * x3 [Variable to solve: x4]
Equation 5: x4 = (1 - 0.050) * x10 [Variable to solve: x10]
In these equations, x1, x2, x3, and x4 represent the fractional yield of fresh water from the first, second, third, and fourth evaporators, respectively. x10 represents the fractional yield of fresh water from the tenth evaporator.
(c) Solution:
To solve the equations derived in part (b), we need to find the values of x1, x2, x3, x4, and x10. These values can be obtained by substituting the known values of salt concentrations and solving the equations simultaneously. Once the values are determined, the fractional yield of fresh water from the process and the weight percent of salt in the solution leaving the fourth evaporator can be calculated.
Learn more about fractional yield
brainly.com/question/29198372
#SPJ11