In a precipitation reaction between FeCl2(aq) and LiOH(aq), 11.3 mL of 0.210 M FeCl2(aq) completly reacted with 34.3 mL of LiOH(aq). What was the molarity of LiOH(aq)
Answers
To determine the molarity of LiOH(aq) in the precipitation reaction between FeCl2(aq) and LiOH(aq), we can use the concept of stoichiometry and the volume and concentration information provided.
The balanced equation for the reaction is as follows:
FeCl2(aq) + 2LiOH(aq) → Fe(OH)2(s) + 2LiCl(aq)
From the balanced equation, we can see that 1 mole of FeCl2 reacts with 2 moles of LiOH.
First, let's calculate the number of moles of FeCl2 using the given volume and concentration:
Moles of FeCl2 = Volume of FeCl2(aq) * Molarity of FeCl2(aq)
= 0.0113 L * 0.210 mol/L
= 0.002373 mol
According to the balanced equation, 1 mole of FeCl2 reacts with 2 moles of LiOH. Therefore, the number of moles of LiOH that reacted can be calculated:
Moles of LiOH = (Moles of FeCl2) / 2
= 0.002373 mol / 2
= 0.0011865 mol
Now, let's calculate the molarity of LiOH(aq) using the volume of LiOH(aq) that reacted:
Molarity of LiOH(aq) = Moles of LiOH / Volume of LiOH(aq)
= 0.0011865 mol / 0.0343 L
= 0.0346 M
Therefore, the molarity of LiOH(aq) is 0.0346 M in the given precipitation reaction.
Learn more about precipitation reaction Visit : brainly.com/question/13016165
#SPJ11
Related Questions
Summarize: If you know the RGB values of two colors of light, how could you calculate the RGB value of a mixture of the two colors? _______________________________________
_________________________________________________________________________
Answers
(105, 200, 0) + (203, 30, 186) = (255, 230, 186)
You can see in the example above that every number in the first color is added to the number in the same position within the second color. If the addition of two numbers goes above 255 then the value maxes out at 255 and stays as is.
When an F atom becomes an F-ion, the Fatom
gains a proton
gains an electron
loses an electron
loses a proton
Answers
When an F atom becomes an F-ion, the F atom gains an electron.
An atom is the smallest indivisible particle of an element. An atom consists of proton, electron and neutron, which are called subatomic particles. An atom becomes charged when it loses or gains an electron. The charged atom is called an ion. An atom loses electrons to become positively charged i.e. a cation while it gains electrons to become negatively charged i.e. an anion. Therefore, when an F atom becomes an F-ion, this means that the F atom gains an electron.Learn more at: https://brainly.com/question/13495616?referrer=searchResults
The Fluoride ion given has a -ve charge onto it. Thus in order to become stable Fluorine gains an electron. Thus option b is correct.
The elements in the periodic table try to attain the stable noble gas configuration in the order to become stable. The noble gas configuration has the elements to be less reactive.
When the atom loses an electron in order to become more stable, a +ve charge has been implemented onto the ion.
When the atom gains an electron to become stable, a -ve charge has been added to the ion.
The Fluoride ion given has a -ve charge onto it. Thus in order to become stable Fluorine gains an electron. Thus option b is correct.
For more information about atom, refer to the link:
https://brainly.com/question/21850455
Explain the concept law of diminishing marginal rate of substitution. What is/are the reason/s why the law of diminishing marginal rate of substitution suggest/s that isoquant must be bent toward the origin?
Answers
The law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
In other words, as the quantity of one good increases, the individual is willing to sacrifice fewer units of the other good to obtain an additional unit of the first good. This reflects a diminishing rate of substitution between the two goods.
The reason why the law of diminishing marginal rate of substitution suggests that isoquants must be bent toward the origin is rooted in the concept of diminishing marginal utility. As more units of a particular input (e.g., labor or capital) are added while holding other inputs constant, the additional output gained from each additional unit of the input will decrease. This diminishing marginal productivity leads to a decreasing MRS.
When isoquants (which represent different combinations of inputs that produce the same level of output) are bent toward the origin, it reflects the fact that as more of one input is used, the amount of the other input that needs to be substituted decreases. This bending signifies the diminishing MRS and captures the idea that a larger quantity of one input can be substituted for a smaller quantity of the other input to maintain the same level of output.
Overall, the law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
To know more about marginal rate of substitution, click here, https://brainly.com/question/30763866
#SPJ11
Solution (a) has a hydrogen ion concentration of 2.7 x 10^-3 and solution (b) has a hydrogen ion concentration of 4.1 M. What are their pH and say if they are acids or bases.
Answers
Answer: a) pH = 2.56 , acidic
b) pH = 2.38, acidic
Explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
\(pH=-\log [H^+]\)
a) \([H^+]=2.7\times 10^{-3}\)
Putting in the values:
\(pH=-\log[2.7\times 10^{-3}]\)
\(pH=2.56\)
Thus as pH is less than 7, the solution is acidic.
b) \([H^+]=4.1\times 10^{-3}\)
Putting in the values:
\(pH=-\log[4.1\times 10^{-3}]\)
\(pH=2.38\)
Thus as pH is less than 7, the solution is acidic.
Methanol (CH4O) is used in the production of many chemicals. Methanol is made by reacting carbon monoxide and hydrogen at high temperatures and pressure.
CO(g) + 2H2(g) ---> CH4O(g)
1) How many molecules of hydrogen are needed to produce 25.O g of CH4O?
2) How many molecules of carbon monoxide are needed to produce 25.O g of CH4O?
3) How many molecules of hydrogen are needed to produce 50.0 g of CH4O?
Answers
1) 1.5625 moles of H2 are needed. 2) 0.78125 moles of CO are needed. 3) 1.5625 moles of H2 are needed to produce 50.0 g of CH4O.
Methanol is produced by reacting carbon monoxide and hydrogen at high temperatures and pressure according to the chemical equation CO(g) + 2H2(g) → CH4O(g). From the equation, we can see that two molecules of hydrogen are required for every one molecule of methanol. Therefore, we can find the number of moles of hydrogen required by dividing the number of moles of methanol by two.
To produce 25.0 g of methanol, we need 1.5625 moles of H2. Similarly, we can find the number of moles of CO required to produce methanol by dividing the number of moles of methanol by one, which yields 0.78125 moles of CO. To produce 50.0 g of methanol, we need 3.125 moles of H2 and 1.5625 moles of methanol.
Learn more about moles here:
https://brainly.com/question/15209553
#SPJ11
Chemical bonds form because: a. The atoms have more energy bonded together than separated b. The atoms have less energy bonded together than separated
Answers
Answer:
A. The atoms have more energy together than separated.
Explanation:
There are several reasons why a chemical bond could take place. Adding things like heat or pressure stimulate the atoms to force a reaction to take place. But when it comes down to it, the intramolecular forces of the electrons pull each other to form chemical bonds (represented by Valence electrons). The stronger these are, the more likely they are going to bond.
Atoms with lower forces typically don't typically bond with other atoms of lower forces.
A gas has a volume of 13.4 L at 17C. What is the volume of the gas at standard temperature?
Answers
Answer:
This law states that the volume and temperature of a gas have a direct relationship: As temperature increases, volume increases, when pressure is held constant. Heating a gas increases the kinetic energy of the particles, causing the gas to expand.
Explanation:
Considering the Charles's law and STP conditions, the volume of the gas at standard temperature is 12.61 L.
Charles's lawCharles's law establishes the relationship between the volume and temperature of a gas sample at constant pressure.
This law says that for a given sum of gas at constant pressure, as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases. That is, the volume is directly proportional to the temperature of the gas.
Mathematically, Charles's law states that the ratio between volume and temperature will always have the same value:
\(\frac{V}{T} =k\)
Considering an initial state 1 and a final state 2, it is fulfilled:
\(\frac{V1}{T1} =\frac{V2}{T2}\)
Definition of STP conditionThe STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C (or 273 K) are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
Volume of the gas at standard temperatureIn this case, you know:
V1= 13.4 LT1= 17 C= 290 K (being 0 C= 273 K)V2= ?T2= 0 C= 273 K (at STP)Replacing in the definition of Charles's law:
\(\frac{13.4 L}{290 K} =\frac{V2}{273 K}\)
Solving:
\(V2= 273 K\frac{13.4 L}{290 K}\)
V2= 12.61 L
Finally, the volume of the gas at standard temperature is 12.61 L.
Learn more about
Charles's law:
https://brainly.com/question/4147359
STP conditions:
brainly.com/question/26364483
brainly.com/question/8846039
brainly.com/question/1186356
#SPJ2
How many grams of sugar must be dissolved in 150 ML of water to make a solution that has a concern traction of .6 g/ml
Answers
Hey there!:
Density = 0.6 g/mL
Volume = 150 mL
Mass = ?
therefore:
D = m / V
0.6 = m / 150
m = 0.6 x 150
m = 90.0 g of sugar
Hope this helps!
a 1) How would you make 1 liter of a 10% NaCl solution from a solid stock? Provide details of what kind of containers you would use.
Answers
To make 1 liter of a 10% NaCl solution from a solid stock, you will require the following materials and containers.MaterialsSolid NaClDistilled water1-Liter volumetric flask250-mL volumetric flask 2-beakersProcedureTo prepare 1 liter of a 10% NaCl solution, the following procedure should be followed:Measure out 100g of NaCl using a balance.
Measure the weight of an empty 250-mL volumetric flask.Add the NaCl to a 250-mL beaker and add a small amount of distilled water to it to dissolve the NaCl.Carefully pour the dissolved NaCl solution into the 250-mL volumetric flask. Add distilled water to the mark on the flask to make up the volume. Stopper the flask and invert it several times to mix the solution.Measure the weight of the 1-Liter volumetric flask.Add the 250-mL volumetric flask solution to a 1-Liter volumetric flask.Add distilled water to the mark on the flask to make up the volume.
Stopper the flask and invert it several times to mix the solution.The final volume of the solution will be 1 liter of a 10% NaCl solution.PrecautionsEnsure the NaCl has completely dissolved before adding more water to avoid making a less concentrated solution.Measure the weight of the volumetric flask before and after adding the solution to calculate the volume of solution that was added.Use distilled water to prepare the solution.
To know more about volumetric flask visit:-
https://brainly.com/question/28997155
#SPJ11
This is the correct answer to
In the experiment, the ______ was intentionally manipulated. It is the independent variable.
The dependent variables that were measured were the ________.
1. Amount of compost.
2. Number of plants and Average height.
I know this helped because I got it correct. GOOD LUCK!!
Answers
Explanation:
1 st one Amount of compost1 st one Amount of compost2nd one Number of plants and Average height.Your Brainly guide
Answer:
amount of compost is the first answer
how is molarity calculated if given moles and volume?
Answers
Answer:
do the math then hope sorry man free answer
Explanation:
Answer:
Molarity is the number of moles of a substance per litre. Thus to calculate the molarity, divide the number of moles with the volume in litres.
For example, 0.2 moles of NaCl is dissolved in water to form a solution of 150 mL.
Volume in litres= 150 ÷1000= 0.15 L
Molarity
= 0.2 ÷0.15
= 1.33 M (3 s.f.)
For more questions on molarity, check out: https://brainly.com/question/2516586
10. As the temperature of a fixed volume of a gas increases, the pressure will _______
answer is increase
Answers
7. Circle the correct word: Electrons contribute to an atom's (mass or volume).
Answers
Answer:
i looked it up on google and i kept seeing mass if that helps, ill copy and paste it
Explanation:Electrons are much smaller in mass than protons, weighing only 9.11 × 10-28 grams, or about 1/1800 of an atomic mass unit. ... Electrons contribute greatly to the atom's charge, as each electron has a negative charge equal to the positive charge of a proton.
Why is heterogeneous nucleation favored over homogeneous nucleation?
Answers
I found this, hope it helps

Heterogeneous nucleation is favored over homogeneous nucleation because it requires a lower energy barrier for the nucleation process. Heterogeneous nucleation involves the formation of a new phase on the surface of an existing foreign material, while homogeneous nucleation occurs spontaneously within a uniform medium.
Heterogeneous nucleation is favored over homogeneous nucleation because it occurs on surfaces or interfaces that are different from the bulk material, providing a lower energy barrier for nucleation to occur.
In contrast, homogeneous nucleation occurs within the bulk material, where there is a higher energy barrier due to the lack of nucleation sites.
As a result, heterogeneous nucleation is more likely to occur and is typically associated with faster and more efficient crystallization processes.
Homogeneous nucleation, on the other hand, can lead to the formation of unwanted impurities and defects in the material due to the high energy required for nucleation.
The presence of the foreign surface in heterogeneous nucleation reduces the overall energy required, making it more likely to occur compared to homogeneous nucleation.
Visit here to learn more about Nucleation:
brainly.com/question/30355004
#SPJ11
Calculate the bond order for one carbon-carbon bond in the benzene molecule, taking σ and π bonding into consideration?
Answers
the bond order for one carbon-carbon bond in the benzene molecule, taking σ and π bonding into consideration will be 1.5.
In the benzene molecule, there are 6 carbon atoms arranged in a hexagonal ring, with alternating single and double bonds. Each carbon atom is covalently bonded to two other carbon atoms, with one single bond and one double bond.
To calculate the bond order for one carbon-carbon bond in benzene, we need to take into account both the σ (sigma) and π (pi) bonding. The σ bond is formed by the overlap of two atomic orbitals along the internuclear axis, while the π bond is formed by the overlap of two atomic orbitals perpendicular to the internuclear axis.
In the case of benzene, each carbon-carbon bond has one σ bond and one π bond. The total bond order for each bond can be calculated as the sum of the bond orders for the σ and π bonds. The bond order for a σ bond is 1, while the bond order for a π bond is 0.5.
Therefore, the bond order for one carbon-carbon bond in the benzene molecule is:
Bond order = (σ bond order) + (π bond order) = 1 + 0.5 = 1.5
So the bond order for one carbon-carbon bond in the benzene molecule is 1.5.
Learn more about carbon here:
https://brainly.com/question/3049557
#SPJ4
which of the following statements is true in a reaction system at equilibrium? a. the number of collisions per unit time between reactants is equal to the number of collisions per unit time between products. b. the equilibrium constant is zero. c. reactants are reacting to form products at the same rate as products are reacting to form reactants. d. reactants and products are present in equimolar amounts. e. the product of the concentrations of the products divided by the product of the concentrations of the reactants is always a constant.
Answers
Answer:
the equilibrium constant is zero
Which of the following is a disadvantage of using corn to manufacture ethanol as a fuel additive?
a. Ethanol contains far less energy than other fuels.
b. Ethanol absorbs water and would thus corrode fuel pipelines used in transport.
c. Use of corn as a fuel source drives up the cost of grain worldwide.
d. All of these are disadvantages.
Answers
Option d. All of these are disadvantages of using corn to manufacture ethanol as a fuel additive.
Using corn to manufacture ethanol as a fuel additive has multiple disadvantages, including all of the options mentioned:
a. Ethanol contains far less energy than other fuels: Ethanol has a lower energy content compared to gasoline, which means that vehicles running on ethanol-blended fuel may have reduced fuel efficiency and mileage.
b. Ethanol absorbs water and would thus corrode fuel pipelines used in transport: Ethanol has a hygroscopic nature, meaning it readily absorbs water from the environment. This characteristic can lead to corrosion and damage to fuel pipelines and storage tanks, which can be a logistical and maintenance challenge.
c. Use of corn as a fuel source drives up the cost of grain worldwide: Corn is a primary feedstock for ethanol production, and using it for fuel purposes can create increased demand and competition with food production. This increased demand can lead to higher prices for corn and other grains, affecting food prices and potentially causing food security concerns.
Therefore, the correct answer is option d.
know more about ethanol here:
https://brainly.com/question/281073
#SPJ11
if a 1.885 g cube has a height of 9.30 mm, width of 9.72 mm, and length of 23.70 mm, what is its density in g/ml? report your answer as a number (no units).
Answers
The density of the cube has a height of 9.30 mm is 0.892 g/ml.
What is density?The mass (m) per unit volume (V) of an object is its density.
ρ = m / V
We must first determine the cube's volume (V) using its dimensions in order to get its density:
V = length x width x height
V = 23.70 mm x 9.72 mm x 9.30 mm
V = 2113.18 mm^3
As the units of density are grams per milliliter, the next step is to convert the cube's mass from grams to milliliters:
[13:32, 2/28/2023] +91 95550 65997: 1 gram = 1 milliliter
Therefore, the volume of the cube in milliliters (V) is:
V = 1.885 g
Lastly, we can get the density of the cube using the density formula:
ρ = m / V = 1.885 g / 2113.18 mm^3
ρ = 0.000892 g/mm^3
Therefore, the density of the cube is approximately 0.000892 g/mm^3 or 0.892 g/ml.
Learn more about density, here:
https://brainly.com/question/29775886
#SPJ1
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
I need help getting this done
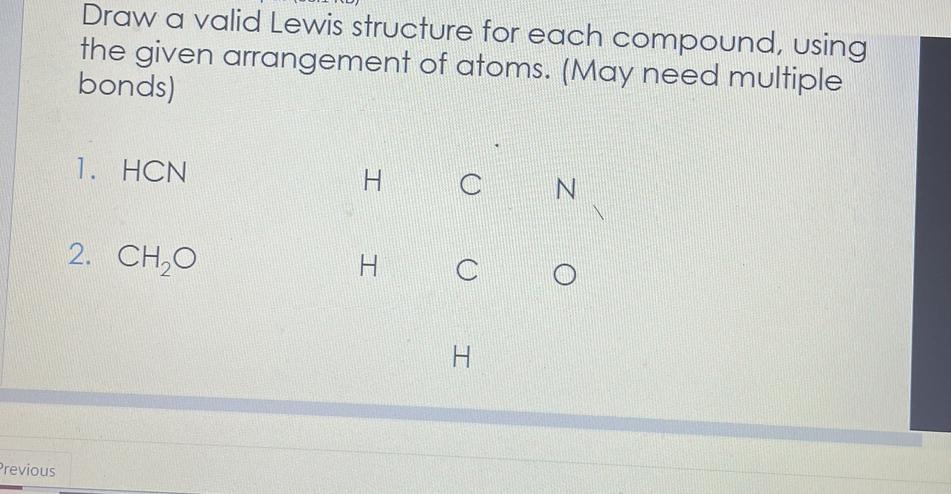
Answers
Answer:
I put the right structures in the pictures for you


HELP ME ASAP PLISSSSS

Answers
Answer:
In this titration, solution P (that contains hydrochloric acid) is added into the conical flask that contains sodium hydroxide drop by drop. The indicator (phenolphthalein) changes color when all the NaOH is completely neutralized.
i) Solution P contains hydrochloric acid. Hydrochloric acid is the chemical that reacts with sodium hydroxide to give salt and water.
This type of reaction is called neutralization.
ii) Under alkaline conditions, phenolphthalein appears pink in color. When the solution becomes neutral or acidic, it is colorless.
So, the color change of the mixture at the end point is from pink to colorless.
iii) same explanation from question i,
HCl (aq) + NaOH (aq) --> NaCl (aq) + H2O (l)
iv) no. of moles = volume (in dm3) x concentration
hence,
no. of moles of NaOH used = 25/1000 x 0.1
= 0.0025 mol
From the equation in (iii), since the mole ratio of HCl : NaOH = 1:1, meaning 1 mole of HCl reacts completely with 1 mole of NaOH.
This also means that the no. of moles of NaOH reacted equals to the no. of moles of HCl needed to react with it, which is 0.0025 mol.
Given the concentration of HCl in solution P is 0.1 mol /dm3. (pls correct me if I misunderstood your comment),
if v is the volume of HCl needed,
0.0025 = 0.1 v
v = 0.025 dm3
also = 25 cm3
Answer:
In this titration, solution P (that contains hydrochloric acid) is added into the conical flask that contains sodium hydroxide drop by drop. The indicator (phenolphthalein) changes color when all the NaOH is completely neutralized.
i) Solution P contains hydrochloric acid. Hydrochloric acid is the chemical that reacts with sodium hydroxide to give salt and water.
This type of reaction is called neutralization.
ii) Under alkaline conditions, phenolphthalein appears pink in color. When the solution becomes neutral or acidic, it is colorless.
So, the color change of the mixture at the end point is from pink to colorless.
Explanation:
In fruit flies the coloration of tan with black stripes (B) is dominant to a solid black i body color (b). A biologist crosses a tan
male with black stripes and a female with a solid black body color.
Which stated observation can lead the biologist to the stated conclusion about the genotype of the male fruit fly in this
cross?
Observation: All the offspring are black
Conclusion: The male has two recessive alleles (bb).
Observation: Half the offspring are black.
Conclusion: The male has two dominant alleles (BB).
Observation: All the offspring are tan with black stripes.
Conclusion: The male has two dominant alleles (BB).
Observation: Most of the offspring are tan with black stripes,
Conclusion: The male has two dominant alleles (BB).
Answers
Answer:If you make a Punnett square, then you have all of the offspring having one dominant allele (the brown body color) and one recessive allele (the back body color).
Explanation:it is expert verified
Rank the following molecules in terms of their carbonyl stretching frequency, v(C=O), in the infrared spectrum. 2-cyclohexenone 2,4-cyclohexadienone cyclohexanone Highest Frequency Carbonyl Stretch Lowest Frequency Carbonyl Stretch 2.4-cyclohexaceenone cyclohexenone 2-cyclohexenone
Answers
The carbonyl stretching frequency in the infrared spectrum depends on the nature of the carbonyl group and the adjacent functional groups or substituents. Based on this, we can rank the given molecules in terms of their carbonyl stretching frequency, from highest to lowest:
2,4-cyclohexadienone > 2-cyclohexenone > cyclohexenone > cyclohexanone
In general, a carbonyl group adjacent to an electron-withdrawing group will have a higher stretching frequency compared to a carbonyl group adjacent to an electron-donating group.
In 2,4-cyclohexadienone, the two carbonyl groups are conjugated with each other and with the double bonds in the ring, resulting in a very high carbonyl stretching frequency. In 2-cyclohexenone, the carbonyl group is conjugated with the double bond in the ring, resulting in a slightly lower stretching frequency.
In cyclohexenone, the carbonyl group is adjacent to a single double bond in the ring, resulting in a lower stretching frequency compared to 2-cyclohexenone. In cyclohexanone, the carbonyl group is not conjugated with any other functional group, resulting in the lowest carbonyl stretching frequency among the given molecules.
For more question on infrared spectrum click on
https://brainly.com/question/5951360
#SPJ11
the electronic configuration of O2−is2s22p6.
Answers
Yes, it is true that the electronic configuration of O2- is 1s2 2s2 2p6.
What is meant by electronic configuration?Arrangement of electrons in orbitals around atomic nucleus is called electronic configuration and describes how electrons are distributed in its atomic orbitals.
When oxygen atom gains two electrons to form an O2- ion, the two electrons occupy the lowest energy level available, which is the 2s orbital. Therefore, the electronic configuration of O2- is the same as that of neon (1s2 2s2 2p6), which has a full outermost shell of electrons. This noble gas configuration makes the O2- ion stable and less likely to react with other elements.
To know more about electronic configuration, refer
https://brainly.com/question/26084288
#SPJ1
When light moves from one substance to another, it can change direction. The following picture shows how this change can make an object in a glass of water seem to bend or even have disconnected pieces.
What is the term for this effect?
A.
transmission
B.
reflection
C.
refraction
D.
absorption
Answers
6. Calculate the new pressure in atm if 2.45 L of a gas at a pressure of 1.20 atm is contracted to a new
volume of 2.20 L
Answers
Answer:
\(\boxed {\boxed {\sf x \approx 1.34 \ atm}}\)
Explanation:
Since we are dealing with temperature and pressure, we must use Boyle's Law. This states that the pressure is inversely proportional to the volume. The formula is:
\(P_1V_1=P_2V_2\)
Where P is the pressure and V is the volume.
We know the original pressure (P₁) of the gas is 1.20 atmospheres and the volume (V₁) is 2.45 liters.
\(1.20 \ atm * 2.45 \ L = P_2V_2\)
We also know the gas is contracted to a new volume of 2.20 liters (V₂) , but we do not know the pressure (P₂). We can use x.
\(1.20 \ atm *2.45 \ L = x * 2.20 \ L\)
We can solve the left side of the equation and multiply.
\(2.94 \ atm *L= x*2.20 \ L\)
We are trying to find the new pressure (x), so we must isolate the variable. It is being multiplied by 2.20 liters. The inverse of multiplication is division, so we divide both sides by 2.20 L.
\(\frac {2.94 \ atm *L}{2.20 \ L}=\frac{x*2.20 \ L}{2.20 \ L}\)
\(\frac {2.94 \ atm *L}{2.20 \ L}=x\)
The units of liters cancel.
\(\frac {2.94 \ atm}{2.20 \ }=x\)
\(1.33636363636 \ atm =x\)
The original measurements have 3 significant figures, so our answer should have the same. For the number we found, that is the hundredth place.
The 6 in the thousandth place tells us to leave the 3 in the hundredth place.
\(1.34 \ atm \approx x\)
The new pressure is approximately 1.34 atmospheres.
At 80. 0°c and 12. 0 torr, the density of camphor vapor is 0. 0829 g/l. What is the molar mass of camphor? report your answer in grams per mole to the nearest whole number.
Answers
The molar mass of camphor in the nearest whole number is 152 g/mol.
Given,
P = 12 torr or 0.01579 atm
1 atm = 760 torr
Density = 0.0829 g/l
R = 0.0821 L.atm/mol.K
T = 273° K + 80° K
T = 353° K
As we know,
PV = nRT
Or, PV = mass × R × T / molar mass
Or, P = mass × R × T / volume × molar mass
Where, mass/ volume = density (d)
P = dRT / molar mass
Now putting the values,
0.01579 = 0.0829 × 0.0821 × 353 / molar mass
Or, molar mass = 152.156 g/mol.
Hence, the molar mass of camphor is 152 g/mol.
The molar mass of a chemical compound is defined because the mass of a pattern of that compound divided by way of the quantity of substance that's the variety of moles in that pattern, measured in moles. The molar mass is a bulk, not molecular, assets of a substance. The molar mass is a mean of many instances of the compound, which regularly vary in mass because of the presence of isotopes. most commonly, the molar mass is computed from the usual atomic weights and is as a result a terrestrial average and a characteristic of the relative abundance of the isotopes of the constituent atoms on the planet. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk portions.To learn more about molar mass, visit: https://brainly.com/question/16377069
#SPJ4
What is an Ore? ufhfjfnf

Answers
Answer:
ore is naturally occuring solid material from which a metal or valuable mineral can be extracted
profitably.
Q1. Sulphur burns in air upon gentle heating with a pale blue flame. It
produces colourless and poisonous sulphur dioxide gas.
a) What are the reactants and products in this reaction? Write as a
word equation.
Answers
Sulfur and oxygen are the reactants in this process, and sulfur dioxide is the end result. Sulfur + Oxygen = Sulfur Dioxide is the word equation for this process.
What is the chemical formula for oxygen and sulfur dioxide?Chemical equation writing. Sulfur trioxide is created when sulfur dioxide and oxygen are combined. Sulfur trioxide, often known as SO3, is the result of the reaction between sulfur dioxide and oxygen (SO2+O2).
The reaction between sulfur dioxide and sulfur oxygen is what kind?This reaction is a combination reaction, which is the type of chemical reaction it is. Balanced Approaches: S and O2 combine to generate SO2 in this reaction of combination. Make sure the number of atoms on either side of the equation is equal by carefully counting them up.
To know more about reactants visit:-
https://brainly.com/question/17096236
#SPJ1
The mathematical expression for h is:
A) mv^2/2
B) v^2/(2g)
C) mg
D) mv
Answers
The mathematical expression for h is v²/(2g) which is based on conservation of energy and the correct option is option B.
The law of conservation of energy states that energy can neither be created nor be destroyed. Although, it may be transformed from one form to another.
Example, when a fruit is falling to the bottom, potential energy is getting converted into kinetic energy.
Conservation of energy implies
KEinitial = PEfinal
mv²/2 = mgh
therefore, h = v²/2g.
Thus, the ideal selection is option B.
Learn more about Conservation of energy, here:
https://brainly.com/question/13949051
#SPJ4