if the ph of a 0.39 m aqueous solution of a weak base is 9.1, estimate the bkb of the base
Answers
The estimated Kb of the weak base is approximately 7.94 × 10^(-5).
How to determine estimated Kb?To estimate the base dissociation constant (Kb) of the weak base, we can use the relationship between the pH, the pKa, and the Kb of a weak acid or base:
pH + pOH = 14
pKa + pKb = 14
where pOH is the negative logarithm of the hydroxide ion concentration [OH⁻].
In this case, we know that the pH of the solution is 9.1. Therefore, we can calculate the pOH:
pOH = 14 - pH = 14 - 9.1 = 4.9
Next, we can use the pKb equation to estimate the Kb of the weak base:
pKb = 14 - pKa
Kb = 10\(^(-pKb)\)
We don't know the pKa of the weak base, but we can assume that it is much smaller than 14 (i.e., the base is weak).
Therefore, we can estimate the pKb as:
pKb ≈ 14 - pH = 14 - 9.1 = 4.9
Kb ≈ 10\(^(-4.9)\) = 7.94 × 10\(^(-5)\)
Therefore, the estimated Kb of the weak base is approximately 7.94 × 10\(^(-5)\).
Learn more about estimated Kb
brainly.com/question/19165803
#SPJ11
Related Questions
Imagine that you have taken a sample of water from a nearby lake. You do a test and find that it has
a pH of 5.6. Adding about of a teaspoon of baking soda to it neutralizes it. What does this tell you
about the baking soda?
Answers
The acidic or alkaline character of a solution is obtained from the value of pH. Here baking soda is a base which neutralizes the acid.
What is pH?The pH of a solution is defined as the negative logarithm of hydronium ion concentration in moles per litre. It can be expressed as:
pH = -log [H₃O⁺]
For acidic solution, the pH will be less than 7 and for basic solution the pH will be greater than 7.
Here the sample of water which has the pH of 5.6 is acidic and it is neutralized by the base baking soda.
Thus we can say that baking soda is a base.
To know more about pH, visit;
https://brainly.com/question/27945512
#SPJ9
A radioactive substance decreases by 65% each hour. Find the hourly decay factor. The hourly decay factor is__
Answers
A radioactive substance decreases by 65% each hour. Find the hourly decay factor. The hourly decay factor is 0.35.
Chemicals in the class of radionuclides (also known as radioactive materials) have unstable atomic nuclei. They become stable by undergoing modifications in the nucleus (spontaneous fission, alpha particle emission, neutron conversion to protons, or the opposite).
A radioactive atom will naturally emit radiation in the form of energy or particles in order to transition into a more stable state. The difference between radioactive material and the radiation it emits must be made.
Learn more about radioactive, here:
https://brainly.com/question/18917411
#SPJ1
calculate the mole in 4.5 molecules of carbon dioxide
Answers
Explanation:
Refer to pic...........

4.51 moles of carbon dioxide
Step by step explanation
To calculate the number of moles in 4.5 molecules of carbon dioxide, we need to use the Avogadro's number, which is the number of particles in one mole of a substance.
Avogadro's number (Nᴀ) is 6.022 x 10²³ particles per mole.
Therefore, we can calculate the number of moles of carbon dioxide as follows:
Number of moles = Number of particles / Avogadro's number
Number of particles of CO2 = 4.5 molecules x 6.022 x 10²³ particles per mole
= 2.711 x 10²⁴ particles
Number of moles of CO2 = 2.711 x 10²⁴ particles / 6.022 x 10²³ particles per mole
= 4.51 moles (rounded to two decimal places)
Therefore, 4.5 molecules of carbon dioxide is equal to 4.51 moles of carbon dioxide
What happens in your cells with cellular respiration?
Answers
What is the difference between the interaction of matter in a compound and the interaction of matter in a mixture?
Answers
Answer:
Matter in compound having chemical bonds while in mixture matter have physical interaction.
Explanation:
We know that element is a substance that is made up of atoms. The atoms of different elements combine to form compound. These are the form of matter that have characteristic properties and having fixed chemical composition. The atoms of different elements are combine to gather through the chemical bond.
For example:
Consider the compound water. It has hydrogen and oxygen. Atoms are bonded together through chemical bond and having fixed proportion. So we can say that their interaction is chemical interaction.
While in mixture different matter combined through the physical interaction.
Atoms of different elements are not chemically bonded together.
For example:
Mixture of sand and water.
Mixture of oil and water.
Below is the electron configuration of calcium: Ca 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 In its reactions, calcium tends to form the Ca2+ ion. Which electrons are lost upon ionization? a. the 4s electrons b. the 1s electrons c. the 3s electrons d. two of the 3p electrons
Answers
When calcium (Ca) becomes an ion (Ca2+), the two 4s electrons are lost. Hence, the correct answer is Option a. the 4s electrons.
The electron configuration of an atom refers to the arrangement of its electrons in its atomic shells. The electron configuration of a neutral atom shows its unexcited state's orbitals, where electrons are placed. A calcium ion (Ca2+) has a +2 charge, indicating that it has two fewer electrons than a neutral calcium atom.
The electron configuration of neutral calcium (Ca) is:Ca 1s2 2s2 2p6 3s2 3p6 4s2When calcium (Ca) ionizes, it loses two electrons from the 4s orbital. Calcium cation (Ca2+) configuration is therefore:Ca2+ 1s2 2s2 2p6 3s2 3p6.
An electron configuration shows how the electrons are distributed within an atom's shells. The notation follows a set pattern and starts with the atom's symbol. For example, the electron configuration of oxygen is 1s² 2s² 2p⁴, which shows the arrangement of electrons in its shells.
Learn more about electronic configuration here:
https://brainly.com/question/29757010
#SPJ11
Question 4 of 10
Which molecule is hexanal?
ОА, НС
ОН
В.
||
Ос. Н?
нсон
D.

Answers
Answer:
Hexanal, once commonly called caproic aldehyde, is a six-carbon straight-chain aldehyde. It is a clear liquid with an atmospheric-pressure boiling point of 131 ºC
Answer:
the answer is B the biggest one
Explanation:
(NEED HELP IMMEDIATELY) In a gene, which base sequence codes for two amino acids?
1. CTAA
2. AG
3. GAGCTC
4. GAA
Answers
Answer:
\(\boxed {\boxed {\sf 3. \ GAGCTC}}\)
Explanation:
Amino acids are the monomers or building blocks of protein. Proteins are created during protein synthesis according to the genetic information or DNA in an organism.
The DNA is translated to messenger RNA in the nucleus. It travels to the ribosome. There, the mRNA is "read off" in threes. Three nucleotides make up a codon. Transfer RNA with the corresponding anticodon and an amino acid attaches to the codon and a chain of amino acid forms.
If 3 nucleotides make 1 codon which makes 1 amino acid, then we need double that or 6 nucleotides to make 2 amino acids.
The only answer choice with 6 nucleotides is Choice 3: GAGCTC
A 3.00 L sample of paint that has a density of 4.65 g/mL is found to contain 33.1 g lead (II) nitride. Determine (A) how many grams of lead ion are in the paint sample? (B) How many ions of lead are in the paint? (C) what is mass percentage of lead in the paint/ What is the ppm?
Answers
1) Grams of lead.
Convert grams of lead (II) nitride to moles of lead (II) nitride
\(molofPb_3N_2=33.1gPb_3N_2\cdot\frac{1molPb_3N_2}{649.61gPb_3N_2}=0.0509lofPb_3N_2\)Convert moles of lead (II) nitride into moles of Pb
\(\text{molesofPb}=0.0509molPb_3N_2\cdot\frac{3\text{molPb}}{1molPb_3N_2}=0.1527\text{molPb}\)Convert moles of Pb into grams of Pb
\(\text{gofPb}=0.1527\text{molPb}\cdot\frac{207.2\text{ g Pb}}{1\text{molPb}}=31.64\text{ g Pb}\)There is 31.64 g of ions of lead in the sample of paint.
describe the manufacture of ethanol from hexane?
Answers
Answer:
This page looks at the manufacture of alcohols by the direct hydration of alkenes, concentrating mainly on the hydration of ethene to make ethanol.
The steps in the ethanol production process include milling the corn to meal, liquefying the meal by adding water and cooking, breaking down starch into sugar, using yeast to ferment the sugar to ethanol, distilling the ethanol by boiling off and condensing it by removing residual water and finally denaturing so that...
cobalt-56 has a half-life of 77.27 days. if we start with a 20 gram sample of co-56, how much will remain after 150 days?
Answers
If cobalt-56 has a half-life of 77.27 days. if we start with a 20-gram sample of co-56, 5.20 grams of cobalt-56 will remain after 150 days.
The given data is as follows:
cobalt-56 has a half-life = 77.27 days
cobalt-56 sample initial mass = 20grams
No of remaining days = 150 days
The exponential decay is used to calculate the given problem.
The general equation used here to calculate here is:
Remaining sample = \(A(\frac{1}{2})^{\frac{t}{h} }\)
= \(20*(\frac{1}{2})^{\frac{150}{77.27} }\)
= \(20*( \frac{1}{2} )^{1.94}\)
= 20 * (0.26)
Remaining sample = 5. 20 grams.
Therefore, we can conclude that 5.20 grams of cobalt-56 will remain after 150 days.
To learn more about half-life problems
https://brainly.com/question/30062135
#SPJ4
compare and contrast the adaptations between plants and animals?
PLS HELP, IT'S A PRESENTATION FOR MY DEBATING LESSONS
Answers
Answer:Plants and animals are living things. They feed, respire, excrete, grow, move, reproduce and are sensitive to their environment
Explanation:
Animals have a central nervous system (CNS) that enables them to respond swiftly to their environmental changes. In contrast, plants lack CNS but have other structural adaptations such as being firmly rooted on the ground, and others can float on water bodies for survival.
The pressure on a 30 mL sample of an ideal gas increases from 400 K to 1600 K at a
constant pressure. The new volume is?
Answers
A 30 mL sample of an ideal gas experiences a continuous pressure increase as the temperature rises from 400 K to 1600 K. The new volume exists 0.015 L.
What is meant by Boyle's Law?Boyle's Law is a gas law that establishes a relationship between pressure and volume of a specific quantity of gas at a fixed temperature.
According to the statement, "The volume occupied by a given gas mass at constant temperature is inversely proportional to the pressure," the volume falls and grows as the pressure changes.
Boyle's law is expressed mathematically as:
Pressure × Volume = constant
or PV = k
If a change in pressure or volume occurs at a constant temperature, the following is true:
P1 × V1 = P2 × V2
The pressure and volume in state 1 are P1 and V1, while the pressure and volume in state 2 are P2 and V2.
In other words, the final pressure divided by the final volume is equal to the product of the starting pressure and the original volume.
P1 = 101.3 kPa = 101,300 Pa (being 1 kPa = 1,000 Pa)
V1 = 30 mL = 0.03 L (1 L = 1,000 mL)
P2 = 202.6 kPa = 202,600 Pa
101,300 Pa × 0.03 L=202,600 Pa × V2
simplifying the above equation we get
\($& V 2=\frac{101,300 P a * 0.03 \mathrm{~L}}{202,600 P a} \\\)
V 2 = 0.015 L
The new volume is 0.015 L.
The complete question is:
The pressure on 30 milliliter of an ideal gas increases from 101.3 kPa to 202.6 kPa at constant temperature. What is the new volume?
To learn more about Boyle's Law refer to:
https://brainly.com/question/1696010
#SPJ1
5. Which of the following are all physical properties of matter?
a. density, color, hardness
b. density, reactions, hardness
c chemistry, freezing point, color
d. lightness, electrons, boiling point
Answers
We wish to determine the moles of
magnesium hydroxide produced when
250 mL of 2.0 M potassium hydroxide
reacts with excess magnesium nitrate.
Mg(NO3)2(aq) + 2KOH(aq) → 2KNO3(aq) + Mg(OH
How many moles of KOH are present
in 250 mL of 2.0 M KOH?
mol KOH
Answers
The number of moles of present in 250 mL of 2.0 M potassium hydroxide may be calculated using the formula: moles = concentration (in M) x volume (in L). The result is the production of 0.25 moles of magnesium hydroxide.
How is a 2M KOH solution created?Pellets of potassium hydroxide weighing 112.22 grammes should be added to the flask. Due to the exothermic process, the water will get hot. (This is the reason for the cold tap water in the bucket to cool down the solution). To completely dissolve the pellets, turn on the stir plate and vigorously mix.
First, we must change the volume from millilitres to litres:
250 mL = 250/1000 L = 0.25 L
Now, we can plug in the values:
moles of potassium hydroxide = 2.0 M x 0.25 L = 0.50 moles
As a result, 250 mL of 2.0 M potassium hydroxide contains 0.50 moles of potassium hydroxide.
The balanced chemical equation indicates: Magnesium is created when 1 mole of Magnesium Nitrate interacts with 2 moles of potassium hydroxide.(hydroxide)2
As a result, the amount of Magnesium(hydroxide)2 generated will be equal to half the amount of potassium hydroxide used:
moles of Magnesium(hydroxide)2 = 0.50 moles potassium hydroxide / 2 = 0.25 moles
To know more about exothermic process visit:-
https://brainly.com/question/31214950
#SPJ1
Answer:0.5
Explanation: the real right answer
Land can also be called:
A) Money and/or profit
B) Natural Resources
C) Human Resources
Answers
A teacher needs to prepare 4.5 L of 1.0 mol/L ehtanoic acid for an investigation. What volume of concentrated 17.4 mol/L ethanoic acid is required?Select one:a.65 mLb.260 mLc.130 mLd.520 mL
Answers
ANSWER
The initial volume of the acid is 260mL
EXPLANATION
Given that;
The final volume of the ethanoic acid is 4.5L
The final concentration of the ethanoic acid is 1.0 mol/L
The initial concentration of the ethanoic acid is 17.4 mol/L
Follow the steps below to find the volume of the ethanoic acid
Step 1; Apply the dilution formula
\(\text{ C1 V1 = C2V2}\)Step 2; Substitute the given data into the formula
\(\begin{gathered} \text{ 17.4 }\times\text{ V1 = 4.5 }\times\text{ 1.0} \\ \text{ 17.4 V1 = 4.5} \\ \text{ Divide both sides by 17.4} \\ \text{ V1 = }\frac{\text{ 4.5}}{\text{ 17.4}} \\ \text{ V1 = 0.260L} \\ \text{ V1 = 260mL} \end{gathered}\)Therefore, the initial volume of the acid is 260mL
Why should you always condition a buret before running a titration?.
Answers
Answer:
Conditioning two or three times will insure that the concentration of titrant is not changed by a stray drop of water.
Explanation:
"Check the tip of the buret for an air bubble. To remove an air bubble, whack the side of the buret tip while solution is flowing".
What is the concentration of KCl if I add 37 grams of K Cl to 0.5 L of distilled water? Give your answer in mols/ /L and in mmols/ L. 8) Blood comprises 7 percent of the body weight in kilograms. How many liters of blood is there be in an 85 kg person?
Answers
The units of concentration in Part A are mols/L and mmols/L, while the unit of volume in Part B is liters
Part A: The concentration of KCl can be calculated by dividing the amount of KCl in grams by its molar mass (in grams/mol) and then dividing by the volume in liters. Given that 37 grams of KCl is added to 0.5 L of distilled water, we divide 37 grams by the molar mass of KCl (74.55 g/mol) to obtain the number of moles.
Then, divide the number of moles by the volume in liters to obtain the concentration in mol/L. To express the concentration in mmols/L, multiply the concentration in mol/L by 1000.
Part B: Blood constitutes approximately 7% of the body weight. To determine the volume of blood in liters for an 85 kg person, we multiply the body weight (85 kg) by the blood percentage (7%) and divide the result by 100.
This calculation provides the volume of blood in kilograms. Since 1 liter of water is equivalent to 1 kilogram, the calculated value represents the volume of blood in liters.
To learn more about concentration.
Click here:brainly.com/question/14469428
#SPJ11
Which statement regarding this equation is TRUE?
4Fe (s) + 3O2 (g) —> 2Fe2O3 (s)
There are 2 reactants.
The coefficient on O2 is 8.
Iron (III) oxide is a gas.
There are 5 atoms of oxygen on the reactant side.
Answers
Answer:
iron (III) oxide is a gas
What volume of a 2.0M NaOH(aq) is needed to completely neutralize 24 milliliters of 0.5M HCl(aq)?
Show numerical setup and answer.
Answers
Answer:
\(V_{base}=6.0mL\)
Explanation:
Hello there!
In this case, by considering that the reaction between sodium hydroxide and hydrochloric acid is in a 1:1 mole ratio of these two reactants, we are able to use the following equation relating the concentration and volume of each one:
\(M_{acid}V_{acid}=M_{base}V_{base}\)
In such a way, by solving for the volume of the base, we will obtain:
\(V_{base}=\frac{M_{acid}V_{acid}}{M_{base}} \\\\V_{base}=\frac{0.5M*24mL}{2.0M}\\\\V_{base}=6.0mL\)
Regards!
I need help please help me please

Answers
Answer: compound thing(picture attached)
Bronze is an alloy made by combining copper and tin. The exact composition of bronze can vary depending on the desired properties, but generally, bronze contains anywhere from 5% to 25% tin.
The reasons why the first humans experimented with making bronze are not fully known, but it is believed that they discovered that adding tin to copper improved its properties, making it harder, more durable, and easier to cast. This would have made it more suitable for weapons, tools, and other objects.
Bronze provided several benefits to early humans. Firstly, bronze tools and weapons were much more durable than those made of pure copper or stone. This made it easier for early humans to hunt, farm, and build, and allowed them to produce more sophisticated and efficient tools. Additionally, bronze objects were more aesthetically pleasing and could be used for decorative purposes. Bronze also played an important role in early trade, as it could be used as a form of currency and was highly valued by many cultures.
In summary, bronze was an important technological advancement in early human history, and its discovery and use played a significant role in the development of human civilization.
Explanation:
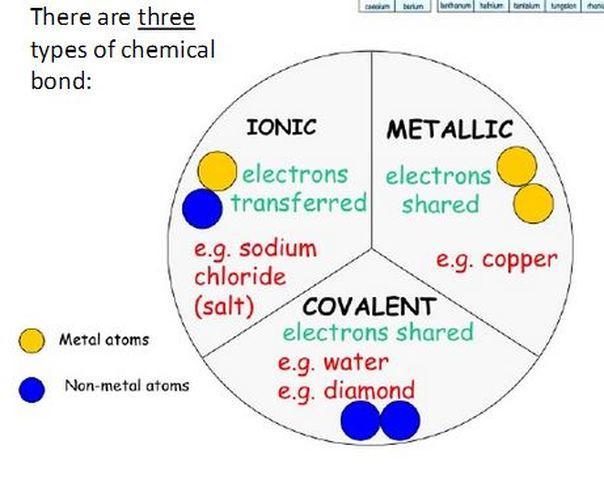
A. The sequence of bases in one DNA strand is given below. Identify the complementary sequence of bases
in the other strand of DNA.
1.
А A
с
T
С
T
G
C
с
А
T
Answers
Answer:
The complementary sequence of bases in the complementary strand of the given single DNA strand AACTCTGCCAT will be:
TTGAGACGGTA
Explanation:
The primary structure of DNA is a double-stranded helix of nucleotides linked to each other by phosphodiester linkages. The two strands of the DNA molecule is complementary to each other. While one strand is linked in the 3' to 5' direction, the other strand is linked in the 5' to 3' direction. The two strands are held together by hydrogen bonds between the nucleotides of the two strands and this is known as base pairing. In base pairing, adenine nucleotide, A, on one strand forms two hydrogen bonds with thymine nucleotdie, T, in the other strand. Also, guanine nucleotide, G, in one strand, forms three hydrogen bonds with cytidine nucleotide, C, in the complementary strand.
Therefore, once the nucleotide sequence of one DNA strand is known, the nucleotide sequence of the complementary strand can easily be deduced.
The complementary sequence of bases in the complementary strand of the given single DNA strand AACTCTGCCAT will be: TTGAGACGGTA
which explains how the nervous system is typically involved in keeping the body in Homeostasis?

Answers
Answer:
c because this is the one hundred all the time
Explanation:

What body system assists the respiratory system by pulling the rib cage down to allow air toflow in?
Answers
Answer:
Diaphragm (a muscle)
Explanation:
the diaphragm moves the allows for more opening as it contracts down. It would most likely be the muacular system since the diaphragm is a muscle.
When the proton number and electron number are unequal, the atom or molecule __________.
Answers
When the proton number and electron number are unequal, the atom or molecule is an ion.
What is proton number and electron number?The total number of protons in the nucleus is known as the atomic number, sometimes known as the proton number, and it is denoted by the letter Z. An electrically neutral atom has exactly as many electrons as its atomic number. Therefore, the nucleus has a total electrical charge of +Ze.For neutrally charged species, the number of electrons in an atom is equal to the atomic number of an element. This indicates that an element has an equal amount of protons and electrons. Consequently, there are 8 electrons in oxygen.Number of Neutrons = Atomic Mass - Atomic NNumber of Protons = Atomic NumberLearn more about proton number and electron number here
https://brainly.com/question/1805828
#SPJ4
65 grams of Potassium Chloride is dissolved to make 450 mL of solution. What is
the MOLARITY of this solution?
Answers
When baking soda is heated it decomposes according to the following reaction:
2 NaHCO3(s) ⇌ Na2CO3(s) + H2O(g) + CO2(g)
If sufficient baking soda is placed in a container and heated to 90°C, the total pressure of the gases is 0. 5451 atm. What is the value of Kp at that temperature?
Answers
The value of Kp at 90°C is zero. This indicates that the decomposition of baking soda at this temperature is essentially complete, and the equilibrium lies far to the right.
We can use the expression for the equilibrium constant Kp, which is given by:
Kp = (P(\(CO_{2}\) ) × P(\(H_{2} O\))) / (P(\(Na_{2} CO_{3}\) ))
where P(\(CO_{2}\)), P(\(H_{2} O\)), and P(\(Na_{2} CO_{3}\)) are the partial pressures of carbon dioxide, water vapor, and sodium carbonate, respectively, at equilibrium.
From the balanced equation, we know that for every 2 moles of \(NaHCO_{3}\)that decompose, 1 mole of \(CO_{2}\) is produced. Therefore, the partial pressure of \(CO_{2}\) can be calculated as:
P(\(CO_{2}\) ) = (1/2) × (total pressure) = 0.2726 atm
Similarly, for every 2 moles of \(NaHCO_{3}\) that decompose, 1 mole of Na2CO3 is produced. Therefore, the partial pressure of \(Na_{2} CO_{3}\)can be calculated as:
P(\(Na_{2} CO_{3}\)) = (1/2) × (total pressure) = 0.2726 atm
Finally, the partial pressure of water vapor can be calculated as the difference between the total pressure and the partial pressures of CO2 and \(Na_{2} CO_{3}\):
P(\(H_{2} O\)) = (total pressure) - P(\(CO_{2}\)) - P(\(Na_{2} CO_{3}\)) = 0.5451 - 0.2726 - 0.2726 = 0.0 atm
This means that there is no water vapor present at equilibrium, and we can assume that its partial pressure is zero. Substituting these values into the expression for Kp, we get:
Kp = (P(\(CO_{2}\)) × P(\(H_{2} O\))) / (P(\(Na_{2} CO_{3}\)))
= (0.2726 × 0.0) / 0.2726
= 0.0
Therefore, the value of Kp at 90°C is zero. This indicates that the decomposition of baking soda at this temperature is essentially complete, and the equilibrium lies far to the right.
Learn more about temperature ,
https://brainly.com/question/11464844
#SPJ4
QUICK IM BEING TIMED
What measurement would answer your question better, “final height of plants” or “rate of growth”?
Choose one answer.
a. Final Height of Plants
b. Rate of Growth
Answers
Answer:
B) rate of growth I'd say
Explanation:
it sounds better I don't think anyone would use height of plants if they want to sound professional
Don’t delete comment or I’ll report

Answers
Answer:
The molar mass of O is 16 g/mol and since we have 2 oxygen molecules we multiply 16 g/mol to get 32 g/mol. Therefore our best option would be option C, 32 g/mol.