Answers
Answer:
The correct answer is 119.
Explanation:
We are given the bond length of XY is 212.
The covalent radius of Y = 93.
Now this means that the bond length(R) is sum of the radio of X and Y Atom.
So, R(XY)=r(X)+r(Y)
212 = r(X)+93
r(X)= 119.
Therefore, the covalent radius of X is 119.
I hope this helps, have a good day! :D
Bond length is the distance between two bonded atoms. The covalent radius of X in XY molecule with bond length 212 and Y with a covalent radius of 93, is 119.
What is covalent radius?Covalent radius is the measure of the atomic size bonded with another atom through covalent bond. Covalent bonds are formed between non-metals with comparable electronegativities.
Bond length of molecule is the distance between the two bonded atom. Mathematically bond length of a covalent compound is the sum of covalent radius of the two bonded atoms.
The bond length of the molecule XY is given as 212 and the covalent radius of Y atom is 93. The bond length 212 is the sum of covalent radius of Y and covalent radius of X.
Covalent radius of X = 212 - 93 = 119.
Therefore, covalent radius of X atom in the XY molecule with bond length 212 and Y atom with covalent radius of 93 is 119 thus, option a is correct.
To find more about covalent radius, refer the link below:
https://brainly.com/question/15117098
#SPJ2
Related Questions
complete the given table by mentioning the quantum numbers for each orbits
Quantum number orbital
2p 3d
azimuthal quantum number ? ?
magnetic quantum number ? ?
Answers
What are the quantum numbers?
The orbital's orientation in space is described by the magnetic quantum number (m). Any number between -l and +l may represent the value of m.
The electron's orbital form is determined by a quantum number called the azimuthal quantum number. Any integer between 0 and n-1 can be used to represent the value of l, and as it rises, the orbital's form becomes more complex.
The quantum numbers that are involved have been shown above.
Learn more about quantum numbers:https://brainly.com/question/16746749
#SPJ1
2. If you travel 7.5 km and walk for 1.5 h. what is your average speed
Show Your Work Actual
Answers
Answer:
5km/h
Explanation:
Given parameters:
Distance = 7.5km
Time = 1.5h
Unknown:
Average speed = ?
Solution:
Average speed is the distance divided by the time taken.
Average speed = \(\frac{total distance }{time taken}\)
Insert the parameters and solve;
Average speed = \(\frac{7.5}{1.5}\) = 5km/h
A galvanic cell consists of a Mg electrode in a 1 M Mg(NO3)2 solution and another metal electrode X in a 1 M X(NO3)2 solution.
The galvanic cell has an E°cell value of 1.61 V. Which of the following elements fits the identity of X. (Use table table 18.1)
Select one:
a.
Pb
b.
Zn
c.
Ni
d.
Fe
e.
Mn
Answers
Answer:
To determine the identity of metal X, we need to compare the standard reduction potentials of the possible metals with the standard reduction potential of the Mg half-reaction.
From Table 18.1, we can find the standard reduction potentials for each of the metals listed:
Pb: -0.13 V
Zn: -0.76 V
Ni: -0.25 V
Fe: -0.44 V
Mn: -1.18 V
The reduction half-reaction for the Mg electrode is:
Mg2+ + 2e- → Mg E° = -2.37 V
The overall reaction for the galvanic cell is:
Mg(s) + X2+(aq) → Mg2+(aq) + X(s)
The standard cell potential is given by:
E°cell = E°(cathode) - E°(anode)
where the cathode is the reduction half-reaction and the anode is the oxidation half-reaction.
Substituting the given values, we get:
1.61 V = E°(X2+/X) - (-2.37 V)
Simplifying, we get:
E°(X2+/X) = 1.61 V + 2.37 V = 3.98 V
Comparing E°(X2+/X) with the standard reduction potentials in Table 18.1, we see that only zinc (Zn) has a reduction potential that is more negative than 3.98 V. Therefore, the metal X is zinc (Zn).
Therefore, the answer is (b) Zn.
Please help me! I don't understand this at all. All the info is in the picture. Thank you so much!!!

Answers
Answer:
H₂S + Cl₂ —> S + 2HCl
Explanation:
? + Cl₂ —> S + 2HCl
To balance the equation above, we must recognise what atoms are present in the products.
The products contains S, H and Cl.
Thus, S, H and Cl must also be present in the reactants.
Considering the equation given above, we can see clearly that H and S is missing in the reactants.
H and S together as a compound is expressed as H₂S.
Now, we shall input H₂S into the equation to obtain the complete equation. This is illustrated below:
? + Cl₂ —> S + 2HCl
H₂S + Cl₂ —> S + 2HCl
Next, we shall verify to see if the equation is balanced.
There are 2 atoms of H on both sides of the equation.
There are 2 atoms of Cl on both sides of the equation.
1 atom of S exist on both sides of the equation.
Thus, the equation is balanced.
Based upon the ion charge of the following polyatomic ions, predict the formula for the following compounds.
sulfate = SO42
phosphate = PO43
hydroxide OH-
sodium hydroxide
O Na(OH)2
O Na(OH)3
O Na₂OH
O NaOH
Answers
Answer:
D.) NaOH
Explanation:
Sodium always forms the cation, Na⁺.
Hydroxide is always written as OH⁻.
The compound should have an overall charge of 0 (be neutral). As you can see, the charges perfectly balance out (+1 + (-1) = 0). Therefore, there only needs to be one atom of each ion. The ionic compound is thus NaOH.
How many atoms of he gas are present in a 450 ml container at 35°c and 740 mmhg?
Answers
Answer:
To answer this question, we will need to use the gas law which states that:
PV = nRT where:
P is the pressure of the gas = 740 mmHg = 98658.550357 Pascal
V is the volume = 450 ml = 0.45 liters
n is the number of moles that we will calculate
R is the gas constant = 8.314 J/(K. mol)
T is the temperature = 35 celcius = 308 degrees kelvin
Substitute with these values in the above equation to get the number of moles as follows:
98658.550357 * 0.45 = n * 8.314 * 308
n = 17.3375 moles
Each mole contains Avogadro's number of atoms, therefore:
number of atoms = 17.3375 * 6.02 * 10^23 = 1.0437 * 10^25 atoms
homogenous and heterogenous catalyst1- state the definition of each one. 2- describe in detail the mechanism of each one. 3- give example of 2 industrial uses per catalyst type with balanced equations for the reactions each catalyzes and the catalyst name.
Answers
1) Homogeneous catalysts are those that occupy the same phase as the reaction mixture (generally liquid or gas), while heterogeneous catalysts occupy a different phase. Normally, heterogeneous catalysts are solid compounds that are added to liquid or gas reaction mixtures.
For most substances the density of a solid form is slightly greater than the density of liquid form water however is different it is slightly less dense in the solid form than it is in liquid form compare water in the solid form with neon argon in oxygen and proposal reason why solid water is less dense liquid water
Answers
Answer:
Answer: When water freezes, water molecules form a crystalline structure maintained by hydrogen bonding. Solid water, or ice, is less dense than liquid water. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart, which lowers the density.
Explanation:
Which is true of magnetic fields?
A.
They are strongest at each pole, where the field lines are closest together.
B.
They are strongest at the north pole, where the field lines are closest together.
C.
They are strongest at the center of the magnet, where the field lines are farthest apart.
D.
They are strongest at each pole, where the field lines are farthest apart.
Answers
Magnetic lines of force are are strongest at each pole, where the field lines are closest together.
What are magnetic fields?A magnetic field is the region around a magnet where the influence of the magnet can be felt. The magnetic line of force show the intensity as well as the direction of the magnetic field.
The fact that is true for all magnetic fields is that they are strongest at each pole, where the field lines are closest together.
Learn more about the magnetic field: https://brainly.com/question/14848188?
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
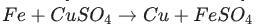
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
Why does the milk flow out of the glass and spread out into thin puddle than staying in the glass or spreading out more across the floor?
A. The particles can break apart but cannot move.
B. The particles can slide past each other, but attractions hold them close together.
C. The particles cannot move, but they can grow in size.
Which is the correct answer A or B or C?
Answers
Chemistry balancing equations - Can this be solved???
find k given [S0₂ 2] =1.50 M, [O₂] = 1.25 M [SO₃] = 3.50 M 2 SO₂ + 1 O₂ ← →2 SO₃
Answers
The value of K is 4.4 represents the equilibrium position of the reaction under the given conditions.
The equilibrium constant (K) for a reaction is a constant value that relates the concentrations of the reactants and products at equilibrium. For the given reaction, the equilibrium concentrations of SO₂, O₂, and SO₃ are given as [SO₂] = 1.50 M, [O₂] = 1.25 M, and [SO₃] = 3.50 M, respectively.
The equilibrium constant (K) for the reaction can be calculated using the equilibrium concentrations of the reactants and products.
K = [SO₃]²/([SO₂]²[O₂])
K = (3.50)²/[(1.50)²(1.25)]
K = 4.48
Therefore, the equilibrium constant (K) for the reaction is 4.48.
To know more about the Balancing equations, here
https://brainly.com/question/7181548
#SPJ1
Tenormin, a member of the group of drugs known as beta-blockers, is used to treat high blood pressure and improve survival after a heart attack. It works by slowing down the heart to reduce its workload. Which atom in Tenormin is the most basic?
Answers
Answer:
Oxygen
Explanation:
In the compound tenormin, there are two highly electronegative atoms capable of accepting electrons; oxygen and nitrogen. Oxygen is more electronegative than nitrogen.
However, the oxygen atom in tenormin is bonded to carbon in a carbonyl bond. Recall that the carbonyl bond is polar and the direction of the dipole is towards the oxygen atom. Looking at the structure of tenormin, it is clear that the electron density of the bond tends towards the oxygen atom of the carbonyl group. Electron density is withdrawn from the adjacent nitrogen atom of the amine group via mesomeric and inductive mechanism towards the more electronegative oxygen atom.
On the other side of the structure, there are two oxygen atoms. These oxygen atoms are more electronegative than nitrogen thus they are more basic.
Hence the oxygen atom is the most basic atom in the compound tenormin.
What is the molar concentration of Zn2+ ions in a solution, if the electrode potential value is 59mV less than the standard electrode potential value at 298 K?
Answers
Molar concentration of Zn2+ions in a solution is 3.481 mol/lit
The electrode potential value is 59mV
Temperature=298k
What is electrode potential?
It is a force of galvanic cell. basically it is the difference between an electrolyte and electrode.equation formed- Zn → Zn2+ + 2e
from Nernst equation-
E=E cell - 0.059 log [Zn2+]
[zn2+]=3.481 mol/lit
hence, Molar concentration of Zn2+ions in a solution is 3.481 mol/lit
Learn more about electrode potential here:
https://brainly.com/question/15417662
#SPJ10
glucose is a six carbon sugar. Albumin is a protein with 607 amino acids. the average molecular weight of a single amino acid is 135 g/mol. there is no reason to run these solutes at the 20 MWCO because
Answers
There is no reason to run these solutes at the 20 MWCO because they are both much smaller than the MWCO of the membrane.
The MWCO (molecular weight cut off) is the molecular weight of a solute at which it will be retained by a membrane during a process such as ultrafiltration or dialysis. If a solute has a molecular weight higher than the MWCO of a membrane, it will be retained and not pass through the membrane. If the molecular weight of a solute is lower than the MWCO, it will pass through the membrane.
In this case, glucose has a molecular weight of 180 g/mol (6 carbons x 12 g/mol per carbon + 6 oxygens x 16 g/mol per oxygen) and albumin has a molecular weight of approximately 81,942 g/mol (607 amino acids x 135 g/mol per amino acid). Both of these solutes have molecular weights that are much lower than 20,000 g/mol, which is a typical MWCO for ultrafiltration or dialysis membranes.
They would both easily pass through the membrane and be lost during the process. Instead, a membrane with a much lower MWCO would be needed if we wanted to retain these solutes during a process such as ultrafiltration or dialysis.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ1
A radical is a reactive intermediate with a single ____________ electron, formed by ____________ of a covalent bond. Allylic radicals are stabilized by ____________ , making them ____________ stable than tertiary radicals. A compound that contains an especially weak bond that serves as a source of radicals is called a radical ____________ . Treatment of cyclohexene with N-bromosuccinimide in the presence of light leads to ____________ by ____________ intermediates.
Answers
1. A radical is a reactive intermediate with a single ____________ electron, formed by ____________ of a covalent bond.
1. A: Unpaired, and homolysis
2. Allylic radicals are stabilized by ____________ , making them ____________ stable than tertiary radicals.
2. A: Resonance, and more
3. A compound that contains an especially weak bond that serves as a source of radicals is called a radical ____________ .
3. A: Initiator
4. Treatment of cyclohexene with N-bromosuccinimide in the presence of light leads to ____________ by ____________ intermediates.
4. A: Allylic substitution by radical
help pls!!! how many moles of H3PO4 form from 8.0 moles of H2O?

Answers
The number of mole of H₃PO₄ that can be formed from the reaction of 2 moles of P₄O₁₀ and 8 moles of H₂O is 5.33 moles
How do i determine the mole of H₃PO₄ formed?First, we shall determine the limiting reactant. Details below
P₄O₁₀ + 6H₂O -> 4H₃PO₄
From the balanced equation above,
1 mole of P₄O₁₀ reacted with 6 moles of H₂O
Therefore,
2 moles of P₄O₁₀ will react with = 2 × 6 = 12 moles of H₂O
From the above calculation, we can see that a higher amount of H₂O is needed to react completely with 2 moles of P₄O₁₀
Thus, H₂O is the limiting reactant.
Now, we shall determine the mole of H₃PO₄ formed from the reaction. Details below:
P₄O₁₀ + 6H₂O -> 4H₃PO₄
From the balanced equation above,
6 moles of H₂O reacted with 4 moles of H₃PO₄
Therefore,
8 moles of H₂O will react with = (8 × 4) / 6 = 5.33 moles of H₃PO₄
Thus, the mole of H₃PO₄ formed is 5.33 moles
Learn more about number of mole:
https://brainly.com/question/13375719
#SPJ1
The addition of 3.15 g of Ba open parentheses OH close parentheses subscript 2 times 8 straight H subscript 2 straight O to a solution of 1.52 g of NH subscript 4 SCN in 100 g of water in a

Answers
The heat that is absorbed by the system is 1363 J. Option B
What is the heat absorbed?We know that in a chemical reaction that there could be the absorption or the evolution of heat. We say that there is the evolution of heat when heat has been lost from the system and there is the absorption of heat when heat has been gained by the system.
Number of moles of the barium hydroxide hydrate = 3.15 g/203 g/mol
= 0.015 moles
Number of moles of the ammonium thiocyanate = 1.52/76 g/mol
= 0.02 moles
If 1 mole of barium hydroxide hydrate reacts with 2 moles of ammonium thiocyanate
0.015 moles of barium hydroxide hydrate reacts with 0.015 * 2 moles/1 mole
= 0.03 moles
Hence the limiting reactant is the ammonium thiocyanate.
Now the heat that is absorbed is;
H = mcdT
m = mass of the water
c = Heat capacity
dT = Temperature change
H = 100 * 4.20 * 3.1
H = 1363 J
Learn more about heat capacity:https://brainly.com/question/28302909
#SPJ1
all organisms begins life as a ________ cell.
Answers
Answer:
All organisms begin life as a single cell
Explanation:
Single cell also did you know 15 minutes can save you 15% more on car insurance

Convert .00612 kg to decigrams.
Answers
0.00612(10000) = 61.2 decigrams
Answer should be 61.2 decigrams.
Hope this helps! :)
Which treatment(s) will help remove contaminants from minerals or from the pipes carrying water from a source? you can select more than one (Water Contamination Gizmos) **ONLY ANSWER IF YOU ACTUALLY KNOW ❗️❗️**
answer choices:
Sedimentation
Disinfection
Filtration
Coagulation

Answers
Sedimentation, filtration, and coagulation are the treatments that will help remove contaminants from minerals or from the pipes carrying water from a source.
Sedimentation is a process in which suspended particles settle out of water. It is one of the most basic techniques for removing particles from water. As particles settle, they become trapped in the bottom of a container or settle to the ground in an outdoor setting
Filtration is a method of removing particles from a fluid. It is a physical or chemical separation method that separates solids from fluids (liquids or gases) by adding a medium through which only the fluid can pass.
Coagulation is the process of using chemicals to remove contaminants from water. By creating a chemical reaction, coagulation destabilizes particles and causes them to clump together. This helps to remove the contaminants from the water.
Disinfection is the process of eliminating or destroying pathogens that cause infection. Disinfection eliminates harmful microorganisms by destroying or inactivating them. The disinfectant is a chemical or physical agent that is used to destroy or inactivate harmful microorganisms.
Know more about Filtration here:
https://brainly.com/question/29756050
#SPJ8
How does an object become electrically charged?will give brainliest
Though the transfer of charges from one object to another
Through the movement of heat from one object to another
Through the transfer of sound from one object to another
Through the movement of water from one object to anoth
Answers
Answer:
It is Though the transfer of charges from one object to another, or (A).
What is the mass of phosphorus in a 63 kg person?
Answers
The mass of phosphorus in a 63 kg person would be 0.63 kg or 630 grams.
Percentage of phosphorus in humansAccording to several research findings, the percentage of phosphorus in the human body is about 1%. This means that the weight of an average human is about 1% phosphorus.
Using the above information, the mass of phosphorus in a 63 kg person can be estimated as follows:
1/100 x 63 = 0.63 kg
Since 1 kg = 1000 grams
Thus, 0.63 kg = 0.63 x 1000
= 630 grams
In other words, the mass of phosphorus in a 63 kg person would be 0.63 kg or 630 grams.
More on phosphorus can be found here: https://brainly.com/question/4622631
#SPJ1
a single covalent bond is made up of
Answers
Answer:
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond.
Explanation:
(copied from Google)
Which substances in the list give me some of the reactants and products in the same condition reaction?

Answers
Answer:
1. CuO, 2. O2, and 3. Cu2O.
Explanation:
You can identify that we can state a synthesis reaction between Cu2O and O2 to form CuO:
\(2Cu_2O+O_2\rightarrow4CuO.\)The reactants are Cu2O and O2, and the product is CuO.
The answer would be 1. CuO, 2. O2, and 3. Cu2O.
What is the mass of 750 atoms of Nitrogen?
Answers
750 x 14,0067 u = 10,505.025 g
if two objects are in contact with each other and object A is at a lower temperature than object B, which of the following statements is correct?
a. the temperature of object A will decrease
b.the temperature of object B will decrease
c.both objects will remain the same temperature
d.heat energy will flow from object A to object B
Answers
Calculate the number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water.
Answers
The number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water is 0.025 moles.
The reaction of potassium with an excess amount of water is:
2K + 2H\(_2\)O \(\rightarrow\) 2KOH + H\(_2\)
To calculate the moles of hydrogen gas first we need to calculate moles of potassium in 2.00g
No. of moles = (mass) / (molecular mass)
The mass given is 2.00 g and the Molecular mass is 39.09 units
∴ No. of moles = (2) / (39.09) = 0.05
From the above reaction, we get that 2 moles of potassium give 1 mole of hydrogen gas. Thus, 0.05 moles of potassium gives 0.025 moles of hydrogen gas.
Therefore, the no. of moles of hydrogen gas produced is 0.025 moles.
To learn more about potassium,
brainly.com/question/13321031
(b) Two compounds, A and B, have the molecular formula C₂H6O. On treatment with Na metal, compound A releases H2 gas and compound B does not.
Can you give a reason to help to explain the observation better?
Answers
The observation that compound A releases H2 gas while compound B does not when treated with Na metal can be explained by considering the structural differences between the two compounds and their ability to undergo specific reactions.
Compound A and compound B both have the molecular formula C₂H₆O, which indicates that they both contain two carbon atoms, six hydrogen atoms, and one oxygen atom. However, the difference lies in the arrangement of these atoms within the molecules. One possible explanation for the observed difference is that compound A is an alcohol, specifically ethanol (CH₃CH₂OH), while compound B is an ether, such as dimethyl ether (CH₃OCH₃). The presence of the hydroxyl group (-OH) in ethanol enables it to undergo a reaction with sodium metal, known as the metal-acid reaction. In this reaction, the metal displaces the hydrogen from the hydroxyl group, forming sodium ethoxide (CH₃CH₂ONa) and releasing hydrogen gas (H₂). On the other hand, ethers like dimethyl ether lack the hydroxyl group and therefore cannot undergo the metal-acid reaction. Consequently, when compound B is treated with sodium metal, no hydrogen gas is released. The ability of compound A to release hydrogen gas while compound B does not when treated with sodium metal can be attributed to the presence of a hydroxyl group in compound A (ethanol), enabling it to undergo a metal-acid reaction, whereas compound B (dimethyl ether) lacks the necessary functional group and thus does not undergo this reaction.
For such more questions on structural
https://brainly.com/question/29117530
#SPJ11
Balance the redox reaction by inserting the appropriate coefficients.
redox reaction:
Fe^{3 + } + NO_{2}^{-} + H_{2}O -> Fe^{2 + } + H^{ + } + NO_{3}^{-}
Fe3++NO−2+H2O⟶Fe2++H++NO−3
Answers
The balanced redox reaction is \(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}+H_{2}O\)
To balance the redox reaction: \(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}\), we need to ensure that the number of atoms and charges are balanced on both sides of the equation.
First, let's balance the atoms. We have one Fe atom on both sides, so it's already balanced. Next, we have two oxygen atoms on the reactant side (from \(NO^{2-}\) and \(H_{2}O\)) and three on the product side (from \(NO^{3-}\)). To balance oxygen, we can add an \(H_{2}O\) molecule to the reactant side:
\(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}+H_{2}O\)
Now, let's balance the charges. On the reactant side, the total charge is 3+ (from \(Fe^{3+}\) ) + 1- (from \(NO^{2-}\)) = 2+. On the product side, the total charge is 2+ (from \(Fe^{2+}\)) + 1+ (from \(H^{+}\)) + 1- (from \(NO^{3-}\)) = 2+. The charges are already balanced.
Therefore, the balanced redox reaction is:
\(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}+H_{2}O\)
By adding an additional H2O molecule to the reactant side, we balanced both the atoms and charges in the equation.
Know more about atom here:
https://brainly.com/question/17545314
#SPJ8