if a sample of 4.50 moles of hydrogen gas was reacted with excess nitrogen, how many moles of ammonia gas would be produced?
Answers
Answer: 3.00
Explanation: got the answer from my teachers notes
According to stoichiometry, if a sample of 4.50 moles of hydrogen gas was reacted with excess nitrogen, 3 moles of ammonia gas would be produced.
What is stoichiometry?Stoichiometry is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample.It is useful in balancing chemical reactions.In the given reaction 3 moles hydrogen gives 2 moles ammonia, thus, 4.5 moles of hydrogen will give, 4.5×2/3=3 moles.
Thus, if a sample of 4.50 moles of hydrogen gas was reacted with excess nitrogen, 3 moles of ammonia gas would be produced.
Learn more about stoichiometry,here:
https://brainly.com/question/9743981
#SPJ6
Related Questions
How many grams of O₂ are required to react completely with 14.6 g of Na to form sodium oxide, Na₂O?
Answers
The balanced chemical reaction is :
\(O_2 + 4Na \ -> \ 2Na_2O\)
Number of moles of Na, \(n = \dfrac{14.6}{23} = 0.635 \ mol\) .
Now, from balance chemical reaction we can see that 1 mole of oxygen reacts with 4 moles of sodium.
So, number of moles of oxygen are :
\(n = \dfrac{0.635}{4}\ mole\)
So, amount of oxygen required is :
\(m = \dfrac{0.635 \times 32}{4}\ gm\\\\m = 5.08 \ gm\)
Therefore, 5.08 gram of oxygen will react with 14.6 gram of sodium.
a rock that can float if placed in water
Answers
Answer:
Anything that has less density than water will float on water.
Explanation:
Come on! It's basic knowledge!
A 3-kg bowling ball rolls at a speed of 5 m/s on the roof of the building that is 75 m tall.
Circle one: KE / GPE / both
Show your work for finding the values of each type of energy the object has:
Answers
A bowling ball has both kinetic energy (KE) and gravitational potential energy (GPE). KE is 75J, GPE is 2,310J.
What is kinetic energy ?Kinetic energy is the energy that an object has when it moves. This is the energy an object has due to its mass and velocity. Kinetic energy is defined as the work required to accelerate an object of a certain mass from rest to its current velocity.Kinetic Energy (KE): The kinetic energy of a bowling ball can be calculated using the equation KE = 0.5 * m * v^2. where m is the mass of the object (3 kg) and v is its velocity (5 m/s). Therefore, the kinetic energy of a bowling ball is 75 J. Gravitational Potential Energy (GPE): both: A bowling ball has both kinetic energy (KE) and gravitational potential energy (GPE). 75J for KE and 2,310J for GPE.To learn more about kinetic energy from the given link:
https://brainly.com/question/26472013
#SPJ1
When an atom loses an electron, it becomes d. ionized. e. a plasma. a. dissociated. b. an isotope. c. sublimated.
Answers
Answer:
When an atom loses an electron it becomes ionized
A 60.00g sample of tetraethyl lead, a gasoline additive, is found to contain 38.43g lead, 17.83g carbon, and 3.74g hydrogen. Find its empirical formula.
Answers
In a 60g sample of tetraethyl-lead, a gasoline is addictive, is found to contain 38.43g lead, 17.83g carbon and 3.74g hydrogen, its empirical formula is PbC₆H₂₀.
What is empirical formula ?The term Empirical formula is defined as the chemical formula of a compound that gives the ratios of the elements present in the compound but not the actual numbers or arrangement of atoms.
The percentage mass of Pb = 38.43/60 × 100
= 64.05 %
The percentage mass of C = 17.83/60 × 100
= 29.71%
The percentage mass of H = 3.74/60 × 100
= 6.23%
Now,
Pb → 64.05/207
= 0.3094
C ⇾ 29.71/12
= 2.475
H ⇾ 6.23/1
= 6.23
Therefore, the ratio is as follows:
Pb : C : H = 1 : 8 : 20
Thus, empirical formula is PbC₆H₂₀.
To learn more about an empirical formula, follow the link;
https://brainly.com/question/14044066
#SPJ9
Name the following hydrate: BaCl2·2H2O(s)
Answers
BaCl2·2H2O(s) is Barium chloride dihydrate
Define hydrate.
A substance that comprises water or its component parts is referred to as a hydrate. Different types of hydrates, some of which were so named before their chemical structure was discovered, varied greatly in the chemical state of the water.
The molecule is a monohydrate if only one water molecule is present. A dihydrate is made up of two molecules of water, etc.
The hydrate form of barium chloride is called barium chloride dihydrate. It performs the function of a potassium channel blocker. It is an inorganic chloride, a barium salt, and a hydrate. It has barium chloride in it.
To learn more about hydrate. :
https://brainly.com/question/27298158
#SPJ4
What is the theoretical yield of this test reaction? Record answer to the nearest whole number. G H2O.
Answers
The theoretical yield is obtained from the limiting reactant in the reaction.
The question is incomplete but I will do my best to help. In a reaction, the theoretical yield is calculated from the limiting reactant in the reaction. Therefore, in order to obtain the limiting reactant, we must first obtain which of the reactants is the limiting reactant.
When that is done, the stoichiometry of the reaction can now be used to calculate the value of the theoretical yield from the number of moles of the limiting reactant.
Learn more about theoretical yield: https://brainly.com/question/2506978
Answer:
360
Explanation:
8.
Which of the following is NOT a desirable property for a material that is to be made ir
window?
A) hard
B) waterproof
C) opaque to light
D) rigid
Answers
what needs to occur in order for elements to form a compound?
Answers
Answer:
When two distinct elements are chemically combined.
Explanation:
Chemical bonds form between their atoms the result is called a chemical compound. Most elements on Earth bond with other elements to form chemical compounds, such as sodium (Na) and Chloride (Cl), which combine to form table salt (NaCl)
What are two qualities of nonmetals? Describe each
Answers
Answer:
In the elemental form, non-metals can be gas, liquid or solid. They aren't shiny (lustrous) and they don't conduct heat or electricity well. Usually their melting points are lower than for metals, although there are exceptions. The solids usually break easily, and can't bend like metals
Explanation:
20) Mg + O2 → MgO
Reaction Type
synthesis (s)
decomposition (D)
single replacement (SR)
double replacement (DR) or combustion (C)
Answers
The given reaction Mg + O₂ → MgO is a type of Synthesis reaction. Hence option (1) is correct.
What is Synthesis reaction ?Synthesis reactions are reactions that occur when two different atoms or molecules interact to form one molecule or compound.
It is also known as combination reaction
In the given reaction Mg and O combines with each other to form one product i.e, MgO. Hence, The given reaction Mg + O₂ → MgO is a type of Synthesis reaction. Thus, option (1) is correct.
Learn more about chemical reaction here ;
https://brainly.com/question/27948961
#SPJ1
Analysis of a sample of an oxide of nitrogen gave 47% of nitrogen.What is the empirical formula of the oxide?
Answers
Answer:
N2O
Explanation:
hope am right.....

a 1.16 mol sample of an element has a density of 13.55 g/cm3. if the sample occupies a volume of 17.17 cm3, what is the molar mass of the element?
Answers
To determine the molar mass of the element, we will use the formula:$$\mathrm{Molar\ mass}=\frac{\mathrm{Density}}{\mathrm{Moles}/\mathrm{Volume}}$$Given:$$\mathrm{Density}=13.55\:\mathrm{g/cm^3}$$$$\mathrm{Moles}=1.16\:\mathrm{mol}$$$$\mathrm{Volume}=17.17\:\mathrm{cm^3}$$.
Given that, density = 13.55 g/cm3molar mass = ?mass of sample = density x volume$$\therefore \ \text{mass of sample} = \mathrm{density} \times \mathrm{volume}$$$$\Rightarrow\mathrm{mass} =13.55 \:\mathrm{g/cm^3} \times 17.17 \:\mathrm{cm^3}=232.7315\:\mathrm{g}$$Now, Molar mass is given by,$$\mathrm{Molar\ mass}=\frac{\mathrm{Density}}{\mathrm{Moles}/\mathrm{Volume}}$$$$\Rightarrow\mathrm{Molar\ mass}=\frac{13.55\:\mathrm{g/cm^3}}{1.16\:\mathrm{mol}/17.17\:\mathrm{cm^3}}=103.1\:\mathrm{g/mol}$$Therefore, the molar mass of the element is 103.1 g/mol.
The given information is about the element's sample. The formula for molar mass is used to find out the unknown molar mass of the given element.
To know more about molar mass visit:
https://brainly.com/question/31545539
#SPJ11
lead is a toxic metal that affects the central nervous system. a pbpb-contaminated water sample contains 0.0013 %pb%pb by mass.
Answers
0.0013% = 0.0013 g/100 ml water, or 1.3 mg/100 ml water
so for 145 mg, you need 145×100/1.3 = 11154 ml water or 11.154 L of water. Lead is a toxic metal that affects the central nervous system.
Extensively talking, the frightened gadget is organized into essential parts, the central nervous system(CNS) and the peripheral nervous system (PNS). The CNS is the processing center of the frame and includes the mind and the spinal cord. Both of these are protected through 3 layers of membranes referred to as meninges.
The principal anxious gadget is the frame's processing center. The brain controls most of the capabilities of the frame, inclusive of cognizance, movement, thinking, speech, and the five senses of seeing, listening to, feeling, tasting and smelling. The spinal twine is an extension of the mind.
The brain is what controls all the frame's features. The spinal twine runs from the brain down via the again. It carries threadlike nerves that department out to each organ and frame part. This network of nerves relays messages from side to side from the mind to distinctive parts of the body.
Learn more about central nervous system here:- https://brainly.com/question/2114466
#SPJ4
Compute the number of ions in 58.5 g oh nacl
Answers
Answer: Explanation:
58.5 g of Na Cl = 1 mole 5.85 g ofNaCl = 1 X 5.85/58.5 = 0.1 mole Each molecule of NaCI contains one Na ion and one Cl ion = 2 ions Total moles of ions in 0.1 mole of NaCI = 2 x 0.1 = 0.2 moleswhat volume in ml of concentrated sulfuric acid (18.0)m h2so4) is needed to prepare 9.0l of a 1.00m solution?
Answers
You need 163.8 mL of concentrated sulfuric acid (18M) to prepare 9.0 L of a 1.00M solution.
The given problem can be solved using the molarity equation which is:
Molarity (M) = moles of solute (n) / liters of solution (L)
The balanced chemical equation for sulfuric acid (H2SO4) is:H2SO4 → 2H+ + SO42
The molecular weight of H2SO4 is 98g/mol.
Hence, 18M H2SO4 solution contains 98g/Liter.98g / 18M = 5.44 g/mL5.44 g/mL x 1000 mL/L = 5440 g/L5440 g / 98 g/mol = 55.102M
Concentrated sulfuric acid is 18M.
So, the volume of concentrated sulfuric acid (18M H2SO4) that is needed to prepare 9.0 L of 1.00M solution can be determined as follows:
Number of moles of H2SO4 in 9.0 L of 1.00M H2SO4 solution = 1.0 mol/L × 9.0 L = 9.0 mol
Total number of moles of H2SO4 in the final solution (1.00M) can be calculated as:9.0 mol / 55.102 mol/L = 0.1638 L = 163.8 mL (rounded to 2 decimal places)Therefore,
To know more about "Sulfuric acid" refer here:
https://brainly.com/question/1107054#
#SPJ11
Which of the following is represented by the number of significant figures in a quantity? A) estimation B) precision C) accuracy D) scientific notation
Answers
Answer: the answer is B estimation
Explanation:
i just took a test
The one that is represented by the number of significant figures in a quantity is scientific notation. The correct option is D.
What is scientific notation?Scientific notation is a method of expressing numbers that are either too large or too small to be written in decimal form.
In the United Kingdom, it is known as scientific form, standard index form, or standard form.
The proper scientific notation format is an x 10b, where an is a number or decimal number with an absolute value greater than or equal to one and less than ten, or 1 |a| 10. b is the power of ten required for scientific notation to be mathematically equivalent to the original number.
Scientific notation is represented by the number of significant figures in a number.
Thus, the correct option is D.
For more details regarding scientific notation, visit:
https://brainly.com/question/18073768
#SPJ2
What is an atom of Gold's mass number if it has 111 neutrons?
Answers
Answer:
190
Explanation:
Gold has atomic number of 79, which is the number of protons.
Mass number = #protons + #neutrons = 79 + 111 = 190
the oxidation number of all the element in free state is 0
Answers
Answer:
Zero-Nine
Explanation:
this is becasue these numbers are rather small and if you plug these numbers into an equation you will most likely get 0.
how many layer does the earth have?
Answers
Answer:
The earth is split into four major layers: the crust, the mantle, the outer core and the inner core
Explanation:
he number of gas particles in a container will have the greatest impact on _____.
Answers
The number of gas particles in a container will have the greatest impact on pressure.
The number of gas particles in a container has the greatest impact on pressure because pressure is directly related to the frequency and magnitude of molecular collisions with the container walls.
When gas particles are in motion, they collide with each other and with the walls of the container. These collisions exert a force on the walls, which we perceive as pressure. The more gas particles there are in the container, the greater the number of collisions with the walls per unit of time.
According to the kinetic theory of gases, the pressure of a gas is proportional to the average kinetic energy and the frequency of collisions of the gas particles. Increasing the number of gas particles increases the frequency of collisions, resulting in more forceful and frequent impacts on the container walls. This leads to a higher pressure.
Conversely, if the number of gas particles decreases, there are fewer collisions occurring per unit of time, resulting in a lower pressure. Therefore, the number of gas particles in a container has the greatest influence on the pressure exerted by the gas inside.
To learn more about kinetic energy click here;
brainly.com/question/26472013
#SPJ11
What is the charge of the atom in the diagram below?
A. -3
B. +1
C. 0
D.+3
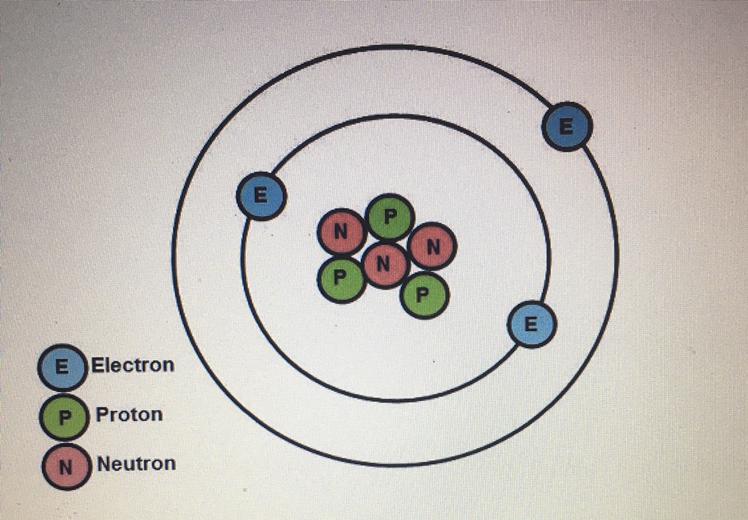
Answers
Answer:
D. +3
Explanation:
have a wonderful day!!
What characteristic of an epic hero does this passage
show?
the ability to embark on a long journey
the ability to receive help from a supernatural force
the ability to speak with confidence
Answers
The ability to speak with confidence shows the characteristic of an epic hero does this passage show. Option C is correct.
What is confidence?
The feeling or emotion or belief that one or a person can have faith in or trust on someone or for something that he or she is doing or about to do and the hero are the inspiration for the society.
In other words a feeling or emotion of self-assurance or self believe arising or rises from an appreciation or motivation of one's own abilities or qualities or performance.
Therefore, ability to speak with confidence shows the characteristic of an epic hero does this passage show. Option C is correct.
Learn more about confidence, here:
https://brainly.com/question/17212516
#SPJ2
Answer:
B
Explanation:
Cuz
Arrange in order of size, with the largest at the top.
Answers
Explanation:
there was any anything to help with
did you attach the picture
Which measurable property of potassium can be used to support this statement: "Matter can be subdivided to the atomic level while retaining its defining characteristics."
A. Temperature
B. Density
C. Mass
D. Volume
Answers
Sep plan and carry out an investigation: plan an investigation using water, a glass, and a bucket to demonstrate that thermal energy is not the same temperature ??
Answers
In bucket the thermal energy is more than the glass
Thermal energy is the energy contained within a system that is responsible for its temperature
The water in the bucket is more than the glass so thermal energy of the bucket is higher than the glass water and the thermal energy is the total amount of kinetic energy of the molecules in the system means in bucket the molecules are more and water are also more so the thermal energy is greater than the glass
Know more about thermal energy
https://brainly.com/question/12455937
#SPJ1
the rate of effusion of co2 gas through a porous barrier is observed to be 9.31x 10^-4 mol/h. under the same conditions, the rate of effusion of N2 gas would be _______ mol/h
Answers
Answer:
\(1.167\times 10^{-3}\ \text{mol/h}\)
Explanation:
\(R_1\) = Rate of diffusion of \(CO_2\) = \(9.31\times 10^{-4}\ \text{mol/h}\)
\(R_2\) = Rate of diffusion of \(N_2\)
\(M_1\) = Molar mass of \(CO_2\) = 44.01 g/mol
\(M_2\) = Molar mass of \(N_2\) = 28.0134 g/mol
From Graham's law we have the relation
\(\dfrac{R_1}{R_2}=\sqrt{\dfrac{M_2}{M_1}}\\\Rightarrow R_2=\dfrac{R_1}{\sqrt{\dfrac{M_2}{M_1}}}\\\Rightarrow R_2=\dfrac{9.31\times 10^{-4}}{\sqrt{\dfrac{28.0134}{44.01}}}\\\Rightarrow R_2=1.167\times 10^{-3}\ \text{mol/h}\)
The rate of effusion of the \(N_2\) gas would be \(1.167\times 10^{-3}\ \text{mol/h}\).
what are the type of mixture in calloid, suspension and solution
Answers
Homogeneous and heterogeneous mixture in colloid, suspension and solution
A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension and within the categories of homogeneous and heterogeneous mixtures there are more specific types of mixtures including solutions is alloys, suspensions, and colloids and a solution is a mixture where one of the substances dissolves in the other and the substance that dissolves is called the solute
Know more about mixture
https://brainly.com/question/1203901
#SPJ1
A decomposition of hydrogen peroxide into water and oxygen gas is an exothermic reaction. If the temperature is initially 28˚ C, what would you expect to see happen to the final temperature? Explain what is happening in terms of energy of the system and the surroundings.
Answers
If the decomposition of hydrogen peroxide into water and oxygen gas is an exothermic reaction, we would expect the final temperature to be lower than the initial temperature of 28˚C.
This is due to the fact that energy is released from the system during an exothermic reaction in the form of heat into the surroundings. In other words, the energy of the reactants is more than that of the products, and the excess energy is released into the environment.
As a result, the environment's temperature will rise, while the system's temperature will fall. This indicates that the reaction's final temperature will be lower than its 28° C starting point.
To learn more about an exothermic reaction, follow the link:
https://brainly.com/question/10373907
#SPJ1
Describe the Quantium Theory of electron orbits and clouds.
Answers
Answer:
The electron cloud model describes electrons as the most likely found around the nucleus of an atom. It is also found that electrons could seemingly be everywhere at one time.
Explanation:
Hope this helped