if a buffer is composed of 27.83 ml of 0.226 m acetic acid and 32.77 ml of 0.168 m sodium acetate, how many ml of 0.100 m hcl can be added before the buffer capacity is reached?
Answers
No Hydrochloric acid (HCl) should be added to maintain the buffer capacity.
To determine how many mL of 0.100 M HCl can be added before the buffer capacity is reached, follow these steps:
Calculate the moles of acetic acid (CH₃COOH) and sodium acetate (CH₃COONa) in the buffer.- moles of CH₃COOH = volume (L) × concentration (M)
= (27.83 mL × 1 L/1000 mL) × 0.226 M
= 0.00629 mol
- moles of CH₃COONa = volume (L) × concentration (M)
= (32.77 mL × 1 L/1000 mL) × 0.168 M
= 0.00550 molThe buffer capacity is reached when the moles of acetic acid and sodium acetate are equal. Calculate the moles of HCl needed to reach this point.
- moles of HCl needed = moles of CH₃COONa - moles of CH₃COOH
= 0.00550 mol - 0.00629 mol
= -0.00079 molSince the moles of HCl needed is negative, it means the buffer already has equal moles of acetic acid and sodium acetate.
Therefore, no HCl should be added to maintain the buffer capacity.
Learn more about Hydrochloric acid (HCl): brainly.com/question/28179864
#SPJ11
Related Questions
How do you calculate neutrons of an element???
Answers
Answer:
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z). Hope This Helps
Explanation:
the ph of a 0.060-m solution of hypobromous acid is 4.96. calculate .
Answers
The Ka value for hypobromous acid (HBrO) is approximately 2.8 x 10^-9.
The pH of a solution can be used to calculate the concentration of H3O+ ions, which can then be used to determine the concentration of the acid (in this case, HBrO). The formula to calculate pH is pH = -log[H3O+].
Given that the pH of the solution is 4.96, we can calculate the concentration of H3O+ ions using the equation 10^(-pH) = [H3O+]. Substituting the given pH value, we have 10^(-4.96) = [H3O+].
Calculating this, we find that the concentration of H3O+ ions is approximately 1.07 x 10^(-5) M.
Since HBrO is a weak acid, we can assume that the concentration of H3O+ ions is equal to the concentration of HBrO. Therefore, the concentration of HBrO is also approximately 1.07 x 10^(-5) M.
The equilibrium expression for the dissociation of HBrO is: Ka = [H3O+][BrO-]/[HBrO]. We can substitute the calculated concentration values to obtain Ka = (1.07 x 10^(-5))^2 / (1.07 x 10^(-5)) = 1.07 x 10^(-5) M.
Therefore, the Ka value for hypobromous acid is approximately 2.8 x 10^-9.
Learn more about molarity visit:
brainly.com/question/30404105
#SPJ11
Consider the unit cell of aluminum with aluminum ion on every corner and every face-centered site of the cube.
Question
1. Using the value of ionic radius of aluminum ion r=0.143 nm, the length of each edge of the unit cell can be calculated as what nm?
2.Using atomic weight value of aluminum 27.0, the density of aluminum can be calculated as what g/cm^3
Answers
Answer:
1) 0.4045nm
2) 0.0027096117g/cm³
Explanation:
1) Using the value of ionic radius of aluminum ion r=0.143 nm, the length of each edge of the unit cell can be calculated as what nm?
In the question above, using a face centered cubic structure, we have:
For a face centered site of the cube,
The diagonal = 4r
Where r =Atomic or ionic radius of Aluminum
Let the edge length of the cube be represented by X
Therefore, we have based on Pythagoras theorem,
X² + X² = (4r)²
2X² = 16r²
Divide both sides by 2
X² = 8r²
Find the square root of both sides
X = √8 × r
Since r = 0.143nm
The length of each edge of the unit cell can be calculated as
X = √8 × 0.143nm
X = 0.4044650788nm
Approximately = 0.4045nm
2) Using atomic weight value of aluminum 27.0, the density of aluminum can be calculated as what g/cm^3
Density of an object = Mass of the Object / Volume of the Object.
The object in this question = Cube
Step 1
Volume of a cube = (Length of the cube)³
In the question above, side length of the cube = 0.4045nm
When would convert 0.4045nm to centimeters
= 1 nm = 1 × 10^-7 cm
0.4045nm =
Cross multiply
= 4.045 × 10^-7 cm
Volume of the Aluminum cube =(4.045 × 10^-7cm)³
= 6.618439112 ×10^-20cm³
Step 2
The atomic weight value of aluminum is given as 27.0 in the question
A face centered cubic structure has 4 atoms per unit cell.
1 Atomic mass or weight value = 1.6605 × 10^-24 grams
Hence, the mass of Aluminum is calculated as:
(4 atoms /1 cell )× 1 unit × (27/ 1 atom of Aluminium) × (1.6605 × 10^-24g/ 1 Atomic mass value)
= 1.79334 ×10^-22g
Density = Mass/Volume
= 1.79334 ×10^ -22g /6.618439112 ×10^-20cm³
= 0.0027096117g/cm³
Hi! I’m so confused… what is the spin quantum number?? :0

Answers
Answer:
5 on k-12 that's the answer
Explanation:
Explanation:
nah so easy
it's a direction of electron spinning in the shell
+½ means electron spinning in clockwise direction
-½ means electron spinning in anticlockwise direction
Calculate the mass of 3.4 moles of nitric acid (HNO3). Explain the process or show your work by including all values used to determine the answer. Source StylesFormatFontSize
Answers
\(\boxed{\boxed{\Large\textsf{$\sf mass = 3.4 \times 63.02=214.3$ g (4 s.f)}}}\)
Explanation:The mass of nitric acid (HNO₃) can be determined by considering the relationship between number of moles, mass, and molar mass:
\(\boxed{\large\textsf{$\sf\displaystyle number\ of\ moles\ (mol)=\frac{mass\ present\ (grams)}{molar\ mass\ (g/mol)}$}}\)
Rearranging this formula to find mass, therefore, mass present = number of moles ÷ molar mass:
\(\boxed{\large\textsf{$\sf\displaystyle mass=moles\times molar\ mass$}}\)
Since we are already given the number of moles of HNO₃ present, we just need the molar mass. The molar mass of elements can be found on any standard periodic table, and the molar mass of a compound is just the sum of all the molar masses of the elements in the compound.
∴ molar mass HNO₃ = H (1.008) + N (14.01) + O₃ (3×16.00) = 63.02 g/mol.
Now plugging in all our values into the formula:
\(\boxed{\boxed{\large\textsf{$\therefore \sf mass = 3.4 \times 63.02=214.3$ g (4 s.f)}}}\)
\(\hrulefill\)
To learn more about moles and molar mass:https://brainly.com/question/34932079

CONNECT IT
Relate how a household sponge and water can be used to model
the concept of an unsaturated solution, a saturated solution, and a supersaturated
solution.
Answers
The sponge is unsaturated when it is taking in more water. It becomes saturated when it stops taking in water. It is supersaturated when water starts oozing out from the sponge.
A saturated solution contains just as much solute as it normally hold at a particular temperature. An unsaturated solution contains less solute than it can normally hold at a particular temperature. A supersaturated solution contains more solute than it can normally hold at a particular temperature.
We can use a sponge to model these three scenario as follows;
Water continues to enter into the sponge when it is unsaturated, this continues until the sponge becomes saturated with water and takes in no more water. When the sponge becomes supersaturated, water begins to ooze out from the sponge because it can no longer hold more water.
Learn more: https://brainly.com/question/1527403
__________ is always involved in hydrolysis reactions.
O None of the listed responses is correct.
O Water
O ATP
O H+ and OH-
O Synthesis
Answers
Water is always involved in hydrolysis reactions in the given options.
A hydrolysis reaction: what is it?The term "hydrolysis" refers to the breakage of chemical bonds by the addition of water and represents the reaction of an organic chemical with water to produce two or more new chemicals. Some occurrences of hydrolysis include the formation of hydronium and bisulfate compounds when sulphuric dissolves in water or a salt of a weak acid or base is mixed with water.
What is the term for sugar hydrolysis?Inversion is the mechanism of hydrolyzing sucrose to produce glucose and fructose. Invert sugar is the consequence of the hydrolysis of sucrose, which causes the sign of rotation to alter from dextro (+) to laevo (-).
Learn more about hydrolysis reactions here:
brainly.com/question/16715756
#SPJ4
Five observable properties for separating mixtures are ______________, _______________, ______________, _____________, ______________.
Answers
Five observable properties for separating mixtures are particle size, colour, texture, shape, magnetic attraction.
Mixtures are compounds that are made up of two or more chemical compounds which are not linked to each other chemically. They can be easily separated.
The mixtures can be separated by properties like particle size, colour, density, shape, magnetic attraction.
Sieving can be used to separate compounds based on particle size. Chromatography can be used to separate compounds based on colour as each colour would have differences in properties. Flotation can be used to separate compounds based on density like oil in water. Centrifugation can be used to separate compounds based on shape. Magnetic separation can be used to separate metals from non-metal compounds in a mixture.
To know more about Mixtures
https://brainly.com/question/24898889
#SPJ1
Which of the following describes ice changing the Earth's surface by erosion?
Water freezes in the cracks of a rock causing bigger cracks to form.
A mound of rock is left as glacier ice melts and the glacier retreats
Sediment frozen in the ice of a glacier moves as the glacier moves.
Heavy ice presses on sediment causing the formation of sedimentary rock
Answers
Answer:
I believe it is D, Heavy ice presses on sediment causing the formation of sedimentary rock
Explanation:
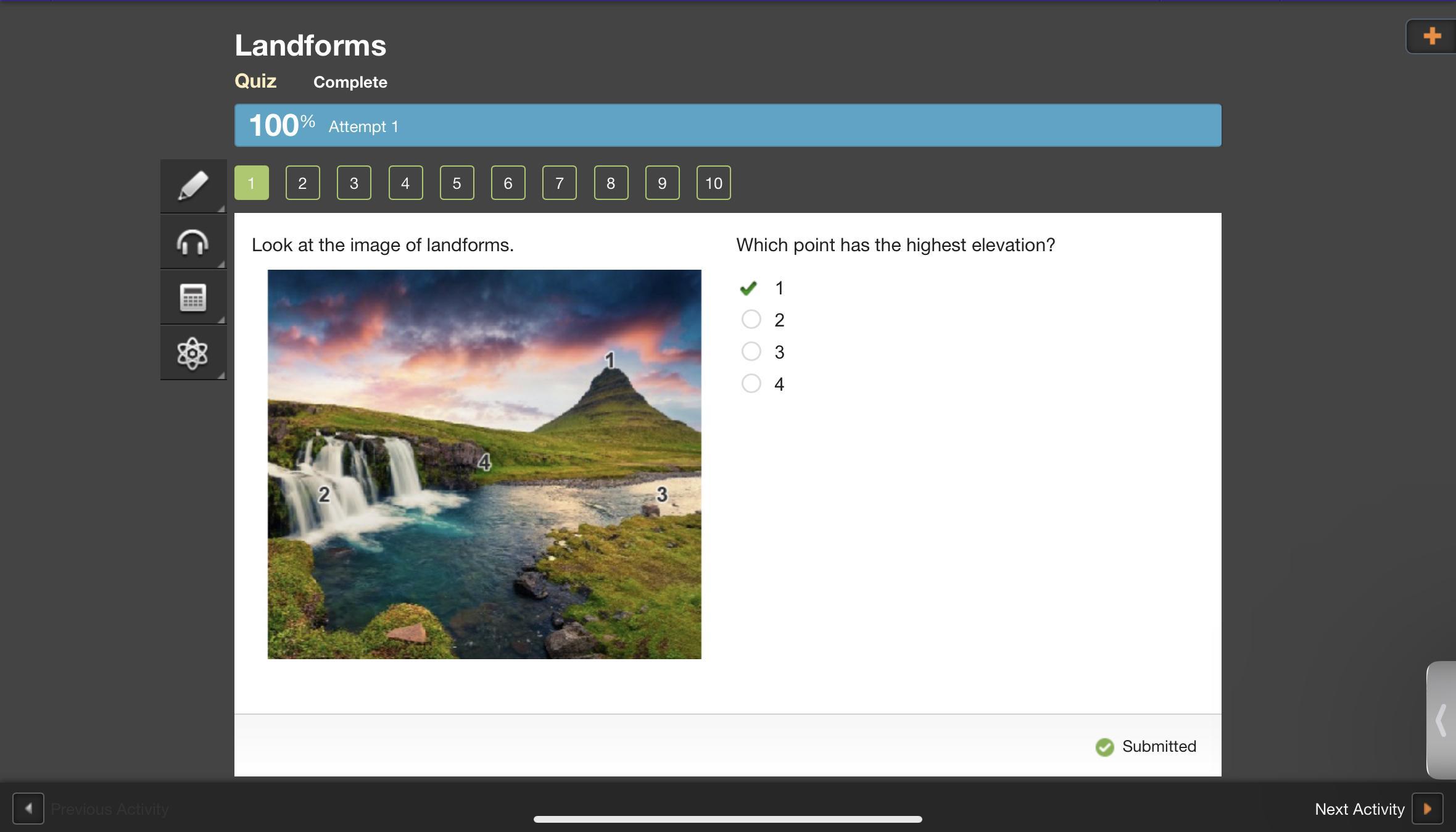
look at the image of landforms which point has the highest elevation
Answers
And I’ll help
Answer: a. 1
Explanation:
Please help If You Are Able To. Where do these go?

Answers
Answer:
Sorry can't help
Explanation:
Cant help!
Humans are not the only animals that pollute the air.
Please select the best answer from the choices provided
F T
Answers
Answer:
TRUE
Explanation:
Humans and many animals are responsible for air pollution. Even natural processess such as excreting feces results in release of methane, CO2 etc which contribute to air pollution
Humans are not the only animals that pollute the air. This statement is True.
What is air pollution ?Substance that alters the natural properties of the atmosphere, whether it be chemical, physical, or biological, is considered an air pollutant. Air pollution can occur inside or outdoors. Common causes of air pollution include motor vehicles, industrial operations, household combustion appliances, and forest fires.
Although there are certain natural processes that can produce air pollution, such as sulfur and chlorine gases from volcanic activity, smoke and ash from wildfires, dust storms, and biological degradation, manmade causes account for the majority of pollution in the atmosphere.
Particles that are both solid and liquid, as well as certain gases that are suspended in the air, are what produce air pollution. These gases and particles can be produced by industries, volcanoes, wildfires, dust, pollen, and vehicle and truck emissions.
Thus, Humans are not the only animals that pollute the air. This statement is True.
To learn more about air pollution follow the link below;
https://brainly.com/question/16357973
#SPJ6
How is protenin in milk broken down in our digestive system
Answers
Answer:
when we drink the milk the digestive system produce protease enzyme to break down the protein of milk.
What is 11.75 millimeters in meters
Answers
Answer:
in meters its 0.01175
Explanation:
Answer:
0.01175 meters
Explanation:
nitric acid (63 g) and sodium hydroxide (60 g) are mixed. determine how many grams of water will form.
Answers
17.99 g of water will formed when nitric acid (63 g) and sodium hydroxide (60 g) are mixed.
When nitric acid and sodium hydroxide are mixed, a neutralisation reaction takes place, with the salt created by the two substances and water serving as the reaction result. The rational response to the statement in this instance is:
Na(OH) + HNO3 ⇒ NaNO3 + H2O
• The formula for HNO3's molar mass is 3xmO + mH + mN, or 3x15.99g + 1g + 14g = 62.97 g/mol.
• The formula for NaOH molar mass is: mO + mH + mNa = 15.99g + 1.00g + 22.99g = 39.98 g / mol.
18g of water and 63g of HNO3
17.99 g of water from 62.97 g of HNO3
62.97 g divided by 18 g and 63 g equals 17.99 g of water.
As a result, 17.99 g of water will form.
To know more about molar mass, visit:
https://brainly.com/question/12127540
#SPJ4
When 0.215 mol of a gas is placed into a 34.25 mL container at 125.0°C, 1
it will exert a pressure on the container. Justin has been asked to
calculate the pressure that this gas will exert on the container in kPa, then
to
go into the lab and measure this pressure.What will he determine is the
pressure of the gas?
Answers
Answer:
205.12 atm
Explanation:
Using the ideal gas law equation:
PV = nRT
Where;
P = pressure (atm)
V = volume (L)
R = 0.0821 Latm/perK)
T = temperature (K)
n = number of moles (mol)
According to the information in this question;
P = ?
V = 34.25 mL = 34.25 ÷ 1000 = 0.03425L
n = 0.215 mol
T = 125.0°C = 125 + 273 = 398K
Using PV = nRT
P = nRT ÷ V
P = (0.215 × 0.0821 × 398) ÷ (0.03425)
P = 7.025 ÷ 0.03425
P = 205.12 atm
Question 7
(01.03 LC)
Which of the following happens to a molecule of an object when the object is cooled?
a) Its temperature increases,
b) It loses kinetic energy.
c) Its size increases.
d) It moves faster.
Answers
Answer:
D
Explanation:
Answer:
D
Explanation:
:)
How many electrons must be gained by nitrogen, N, to achieve a stable electron
configuration?
Answers
Answer:
3 electrons
Explanation:
Nitrate needs 3 electrons to achieve a stable electron configuration
Three is the answer. it needs three to complete its shell
The total charge in a compound must be 0. Explain how this is possible when the anions and cations do not have equal and opposite charges, such as in magnesium chloride and aluminum oxide. ???
Answers
The two chlorine atoms have a total charge of -2 hence the total charge in the compound is zero. Three oxygen atoms each having a charge of -2 making a total charge of -6 hence the total charge in the compound is zero.
In magnesium chloride, magnesium has a charge of +2. Chlorine has a charge of +1. However, in the compound, there are two chlorine atoms and one magnesium atom. As such, the two chlorine atoms have a total charge of -2 hence the total charge in the compound is zero.
In Aluminium oxide (Al2O3), there are two aluminium atoms having a total charge of +6. There are three oxygen atoms each having a charge of -2 making a total charge of -6 hence the total charge in the compound is zero.
Learn more: https://brainly.com/question/6284546
Thomas and Trenton aren't finished with their lab when the bell rings, but they don't want to be late to
lunch. They quickly pour their leftover chemicals down the drain and leave the rest of the lab
equipment they used on the counter, figuring someone in the next class period will probably be using it
anyway.
Answers
Chemicals are not supposed to be poured down the drain.
Should you pour chemicals down the drain?Chemicals should not be poured down the drain. This can cause harm to the plumbing system, sewage treatment plants, and the environment. Dispose of household chemicals properly by taking them to a designated recycling center or household hazardous waste collection event.
Thus the action that have been undertaken by Thomas and Trenton is quite wrong since the chemicals that they have poured down the drain could lead to an environmental hazard.
Learn more about chemicals:https://brainly.com/question/13145357
#SPJ1
Which bonding type results in stronger bonds? What is your evidence?
Answers
Answer:
Covalent bonds are the strongest bonds in nature and under normal biological conditions have to be broken with the help of enzymes. This is due to the even sharing of electrons between the bonded atoms and as with anything equally shared there is no conflict to weaken the arrangement.
Explanation:
Bonding electrons are described as electrons that participate in chemical bonds. Chemical bond, a strong attraction between atoms, ions, or molecules, might be the subject here. Atoms sharing electron pairs form a covalent or molecular connection. An attraction between the atomic orbitals of atoms in a molecule is called a bonding molecular orbital.
Which bonding type results in stronger bonds? What is your evidence?
A covalent bond is created when the difference between two atoms' electronegativities is too small for an electron transfer to occur and produce ions. Bonding electrons are collectively referred to as the electrons that are present between the two nuclei. The "glue" that binds the atoms into molecular structures is the bound pair.
It is the most powerful link. By sharing an electron, the two atoms in such a bond are joined together.
One Hydrogen atom with one valence electron and one Chlorine atom with seven valence electrons, for instance, make up the HCL molecule. In this instance, hydrogen and chlorine share an electron to create a single bond.
To know more about Electron bonding, click on the link below:
https://brainly.com/question/12732708
#SPJ1
What is the purpose of chemistry lab?(solution)
Answers
1. Some reactions will present an actual yield lower than the theoretical yield, and this can occur due to many situations, a couple of the reasons why that we can list is that some reactions don't have the reactants actually reacting to form the products, and another reason why is the loss of reactants in the process for many possible situations.
4. According to the solubility curve, if we have 30 grams of NH4Cl at 50°C, the reaction will be unsaturated
Which are the factors that favor SN2 reactions, as described in the lab lecture?
a) Strong nucleophile, good leaving group, polar protic solver, methyl or primary halide
b) Strong nucleophile, good leaving group, polar aprotic solvent, methyl or primary halide. c) Weak nucleophile, good leaving group, polar aprotic solvent, methyl or primary halide d) Strong nucleophile, poor leaving group, polar aprotic solvent, , tertiary halide.
e) Strong nucleophile, good leaving group, polar aprotic solvent, tertiary halide.
Answers
Strong nucleophile, good leaving group, polar aprotic solvent, methyl or primary halide. The correct answer is option: b.
In an SN2 reaction, a nucleophile attacks the carbon atom to which the leaving group is attached, while the leaving group departs from the molecule. The reaction proceeds in a single step, with the nucleophile and leaving group involved in the transition state. A strong nucleophile is required to attack the carbon atom, and a good leaving group is necessary to depart from the molecule. Methyl or primary halides are preferred substrates because they are less hindered, making the attack by the nucleophile easier. Option b is correct.
To know more about nucleophile attacks , here
brainly.com/question/28325919
#SPJ4
Which of these scientist is know for his work in understanding climate change a : edwin hubble b : christian doppler c : warren washington d : charles kuen kao
Answers
Answer: just trust me its c
Explanation: i dont cap
explain why the containers are heavy
Answers
Answer:
there is no picture so we can't tell why the containers are heavy
True or False (+2 Points Possible each): Write true if the statement is true. If false, replace
the underlined word with the correct word in the space provided.
1. Radiation is the transfer of energy through space.
2. Molten rock rises in Earth's mantle caused by gravity.
3. Fossils provide evidence of sea-floor spreading.
4. Older, denser Earth material sinks below younger material
known as subduction.
5. Convergent boundaries
move plates towards each other
mountains.
is
forming
PLEASE HELP ASAP THIS IS DUE BY MIDNIGHT!

Answers
Answer:
2. Molten rock rises in Earth's mantle caused by (pressure)
Explanation:
i think i just had a similar question
What does the energy hill represent on an energy diagram?
A. The potential energy gained by the products when a reaction
happens
B. The final amount of potential energy of the products of the
reaction
C. The potential energy the reactant's have stored in molecular bonds
D. The additionat potential energy the reactants must gain in order to
React
Answers
Answer:
d
Explanation:
its not a b c
qualitative term for a solution containing solute at a relatively low concentration
Answers
The qualitative term for a solution containing solute at a relatively low concentration is dilute.A solution is a homogeneous mixture of two or more substances.
It consists of a solute that is dissolved in a solvent. There are different types of solutions based on the amount of solute dissolved in a solvent. These types include a dilute solution, a concentrated solution, and a saturated solution. Let's look at the meaning of the dilute solution.
A solution that contains a low concentration of a solute is called a dilute solution. It has a lesser amount of solute dissolved in a solvent. The quantity of solute in a solution is expressed in terms of its concentration. Dilute solutions are often prepared by adding solvent to a concentrated solution. When a solute is added to a solvent, it dissolves to form a solution. As more and more solute is added, the solution's concentration increases.
Concentration is the measure of the amount of solute present in a solution. It can be expressed in qualitative or quantitative terms. Qualitative terms describe a solution as dilute or concentrated based on the amount of solute present in a solution. On the other hand, quantitative terms specify the exact amount of solute present in a solution. This is expressed in units such as parts per million (ppm), molarity (M), and mole fraction (x).
To know more about homogeneous mixture visit:
https://brainly.com/question/30587533
#SPJ11
If an atom has 3 protons, what is its atomic number?
Answers
Answer:
Atomic Number = 3.
Explanation:
The number of protons determines the atomic number. Basically the number of protons is the exact same as the atomic number.
Protons 3 = Atomic number 3
How do scientists calculate the energy and frequency of photons?