if 0.100 mol of an ideal monatomic gas has a pressure of 1.00 atm at 273 k and is cooled at constant pressure until its volume is one third its original volume. what is the amount of work done by the gas?
Answers
When 0.100 mol of an ideal monatomic gas at 273 K and 1.00 atm pressure is cooled at constant pressure until its volume becomes one third of its original volume, the amount of work done by the gas can be calculated.
The work done by a gas can be determined using the equation:
Work = -PΔV,
where P is the pressure and ΔV is the change in volume. In this case, the pressure is constant throughout the process. The initial volume, V1, is not given, but the final volume, V2, is one third of the original volume. Therefore, ΔV is calculated as V2 - V1 = -2/3V1.
Since the gas is cooled at constant pressure, the temperature remains constant as well. According to the ideal gas law, PV = nRT, where n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin. Rearranging this equation gives V = (nRT)/P.
By substituting the values into the equation, V1 can be expressed as (0.100 mol * 0.0821 L·atm/mol·K * 273 K) / 1.00 atm = 2.243 L. Therefore, V2 becomes 2.243 L / 3 = 0.748 L.
Plugging these values into the work equation, we get Work = -(1.00 atm) * (-2/3)(2.243 L) = 1.495 atm·L. The negative sign indicates work done by the gas, and the result is positive, meaning the gas is performing work on its surroundings.
To learn more about work done click here: brainly.com/question/3902440
#SPJ11
Related Questions
Ca(s) + O2(g) -
O A. Cao(s)
B. Ca2O(s)
O C. 2Ca(s) + O2(g)
O D. CaO2(s)
Answers
Answer:
bbbbnnnnnnnnnnnnnnn
Explanation:
Give two differences between the physical properties of the elements in Group 1 and those of the transition elements. [2 marks
Answers
Due to their stronger metallic bonding and more compact atomic structure, the transition elements have higher melting and boiling temperatures and are often denser than the alkali metals.
What are group one metals' two physical characteristics?Elements from Group 1 have similar properties. All of them are supple silver metals. These metals are extremely reactive and have low melting temperatures due to their low ionisation energy. As you descend the chart, this family becomes more reactive.
What are the transitional elements?The d orbitals of transitional elements are only partially filled. A transition element is defined by IUPAC as an element that may form stable cations and has an electron d subshell that is only partly filled.
To know more about transition elements visit:-
https://brainly.com/question/1948991
#SPJ1
Part B: Short Answer - Answer the following questions
6. Why are the elements so important?
7. The transition metal Cobalt has an atomic number of 27 and an atomic mass of approximately 59
AMU. How many neutrons does it have? Show all your calculations for full marks.
I
28
tv
I need help fast 50 points

Answers
Answer:
7. 32neutrons
Explanation:
mass number is equal to number of protons plus the number of neutrons
the atomic number is also known as the number of protons
therefore 59-27=32
eugenol can also be isolated from cloves using extraction with co2. a. true b. false
Answers
b. false
Eugenol is typically extracted from cloves using methods such as steam distillation or solvent extraction, but not with CO2 extraction. CO2 extraction is a technique commonly used to extract essential oils from various plant materials, but it is not the preferred method for isolating eugenol from cloves.
To know more about Eugenol refer here
https://brainly.com/question/31424314#
#SPJ11
Individual solute particles are broken apart from the solid by these particles. * a Solution b Insoluble c Solvent d Compounds
Answers
Answer:
Individual solute particles are broken apart from the solid by the;
c. Solvent
Explanation:
A solution is the homogeneous mixture that is made up of two or more substances formed by dissolving a substance which can be a solid, liquid or gas in another substance known as the solvent which normally the larger part of the fraction of the solution than the solute and can also be a solid, liquid or a gas
In a solution the solvent particles serves to brake of and disperser parts of a solid solute to form a more or less homogeneous mixture
Therefore, the solute particles are broken by the solvent particles in a solution
A liquid is allowed to evaporate and leaves no residue. can you determine whether it was an element, a compound, or a mixture?
Answers
A liquid is allowed to evaporate and leaves no residue. It can not be determined whether it was an element, a compound, or a mixture.
A water vapor molecule stays roughly 10 days in the atmosphere after it has evaporated. Water vapor starts to cool back down as it ascends higher in the atmosphere. The water vapor condenses when it becomes cold enough, turning it back into liquid water. Eventually, individual water droplets will condense to create clouds and precipitation.
It is not possible to determined whether it would be an element, a compound and mixture because the size of the particle will be too less.
A method for separating homogenous mixtures with one or even more dissolved salts is called evaporation. The procedure separates the liquid from the solid components. Usually, the procedure entails heating the combination until there is no more liquid is present.
Therefore, it can not be possible to determined whether it was an element, a compound, or a mixture by evaporation.
To know more about liquid
https://brainly.com/question/25579356
#SPJ4
Which chemical condition describes the electrons in a water molecule being shared unequally between the hydrogen and oxygen atoms? ionic noncovalent polar hydrophobic
Answers
The chemical condition that describes the electrons in a water molecule being shared unequally between the hydrogen and oxygen atoms is called polar covalent bonding.
In polar covalent bonds, the electrons are unequally shared due to the electronegativity difference between the atoms involved. In the case of a water molecule, oxygen is more electronegative than hydrogen, causing the oxygen atom to attract the shared electrons more strongly.
As a result, the oxygen atom becomes slightly negatively charged while the hydrogen atoms become slightly positively charged. This polarity gives water its unique properties, such as its ability to form hydrogen bonds and its high surface tension.
In summary, that this describes the unequal sharing of electrons in a water molecule due to the electronegativity difference between hydrogen and oxygen atoms.
To know more about covalent bond visit:
https://brainly.com/question/3447218
#SPJ11
Using the drop-down menus, complete the table to
summarize the properties of the different
subatomic particles
Particle
Charge
Location
Approximate
mass (amu)
Proton
+1
Nucleus
A
A
Neutron
0
Nucleus
B
DONE
Electron-1
Orbitals
С
Intro
8 of 23
Answers
Approx mass(amu) of Particles proton, neutron and electron is 1, 1, 0.
Electrons are found outside the nucleus in electron shells, not inside the nucleus. Therefore, the location of electrons should be listed as "Extracellular space (outside nucleus)." The approximate mass of an electron is about 1/1836 amu, which is negligible compared to the mass of a proton or neutron. Therefore, the mass of an electron is usually listed as "negligible" or "approximately 0."
Protons are positively charged subatomic particles found in an atom's nucleus. A proton has a charge of +1 and an approximate mass of 1 atomic mass unit (amu). The atomic number of an element is determined by the number of protons in its nucleus.
Neutrons are neutral subatomic particles found in an atom's nucleus. A neutron's charge is zero, which means it has no charge. A neutron has an approximate mass of 1 amu, which is similar to the mass of a proton. The number of neutrons in an atom's nucleus can vary, resulting in isotopes of the same element.
For more question on electron click on
https://brainly.com/question/371590
#SPJ4
Correct question should be
Using the drop-down menus, complete the table to summarize the properties of the different subatomic particles. (options are zero and one for a, b, and c)
Click on image for reference

Is boiling point and melting point are physical properties? (Answer quicklyyyyy)
Answers
A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity.
Consider the fermentation reaction of glucose:
A 1.00-mole sample of C6H12O6 was placed in a vat with 100 g of yeast. If 32.3 grams of C2H5OH was obtained, what was the percent yield of C2H5OH?
A) 35.1%
B) 17.5%
C) 100%
D) 32.3%
E) none of these
Answers
The fermentation reaction of glucose produces C\(_2\)H\(_5\)OH and CO\(_2\) according to the following equation:
C\(_6\)H\(_{12}\)O\(_6\) → 2C\(_2\)H\(_5\)OH + 2CO\(_2\)
The molar mass of glucose (C\(_6\)H\(_{12}\)O\(_6\)) is 180.16 g/mol, so 1.00 mole of glucose corresponds to 180.16 g.
To find the theoretical yield of C\(_2\)H\(_5\)OH, we need to use stoichiometry. From the balanced equation, we see that 1 mole of glucose produces 2 moles of C\(_2\)H\(_5\)OH. Therefore, 1.00 mole of glucose should produce 2.00 moles of C\(_2\)H\(_5\)OH.
The molar mass of C\(_2\)H\(_5\)OH is 46.07 g/mol, so 2.00 moles of C\(_2\)H\(_5\)OH corresponds to 92.14 g.
The actual yield of C\(_2\)H\(_5\)OH obtained is 32.3 g.
The percent yield is calculated as (actual yield/theoretical yield) x 100%. Therefore, the percent yield of C\(_2\)H\(_5\)OH is:
(32.3 g/92.14 g) x 100% = 35.1%
So the answer is (A) 35.1%.
Learn more about yield at: https://brainly.com/question/14714924
#SPJ11
A compound is 2.00% h by mass, 32.7% s by mass, and 65.3% o by mass. what is the correct empirical formula for this compound? hso h2s3o4 h2so4 h3s2o5
Answers
The correct empirical formula for this compound is H2So4 (sulfuric acid).
The molar mass of H = 1,O = 16, S = 32
Thus, the number of moles of H, O, and s are:
H = 2.1 / 1 = 2.1
O = 65.3 / 16 = 4.08
S = 32.6 / 32 = 1.02
Upon dividing the number of moles of each component by the smallest value i.e. 1.02 and rounding the number to the nearest integer, we get:
H = 2 , O = 4, S = 1
Hence, the required empirical formula is: H2SO4.(Sulfuric acid)
A colorless, greasy liquid is sulfuric acid. When the heat is released, it dissolves in water. Metals and tissue are both corroded by it. On contact, it will scorch most organic materials, including wood, but it is unlikely to start a fire. 15 pounds per gal of density of mass . Inhalation can have negative health effects after prolonged exposure to low doses or brief exposure to high quantities. In addition to many other purposes, it is used to produce iron and steel, fertilizers, other chemicals, and other products. Rate of onset: Instantaneous Duration: Hours, Days Source/Use/Other Hazard: Battery, dye, paper, glue, metals industries; volcanic gas; poisonous gases when heated.
Learn more about Empirical formula here:
https://brainly.com/question/14044066
#SPJ4
The chart indicates the time, speed, and velocity of five runners.
A 4-column table with 5 rows. The first column labeled runner has entries Garreth, Luigi, Elisa, Jasmine, Logan. The second column labeled total time has entries 45 minutes, 35 minutes, 40 minutes, 35 minutes, 35 minutes. The third column labeled speed has entries 6.2 miles per hour, 5.0 miles per hour, 6.2 miles per hour, 5.0 miles per hour, 5.6 miles per hour. The fourth column labeled velocity has entries 5.5 miles per hour west, 4.3 miles per hour east, 5.5 miles per hour west, 4.0 miles per hour south, 4.0 miles per hour north.
Which best describes the runners?
Garreth and Elisa ran the same distance but not the same displacement, Luigi and Jasmine went the same displacement, and Logan ran faster than Jasmine.
Garreth ran farther than Luigi, Elisa and Jasmine went the same distance, and Logan and Jasmine has the same displacement in the opposite direction.
Garreth and Elisa ran together, Luigi and Jasmine went the same distance, and Logan and Jasmine have the same displacement in the opposite direction.
Garreth ran less than Logan, Elisa and Jasmine went the same displacement, and Luigi and Garreth ran together.
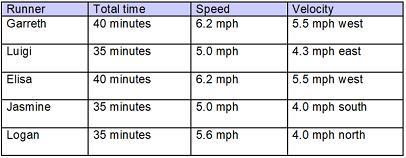
Answers
The true statement is that; Garreth and Elisa ran together, Luigi and Jasmine went the same distance, and Logan and Jasmine have the same displacement in the opposite direction. Option C
What is true from the table?We have to note that the distance has to do with the distance that is covered but the direction is not involved. If we are looking at the displacement, we would be talking about the distance that is covered in a specific direction.
Now we have to put this in perspective as we look at the table, it is clear that we know that the displacement of Logan and Jasmine have the same distance but in opposite direction.
Learn more about speed:https://brainly.com/question/28224010
#SPJ1
Answer:
C
Explanation:
took on eddge
which element is unreactive and has a greater atomic radius than 1
Answers
Answer:
Atomic radii vary in a predictable way across the periodic table. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Thus, helium is the smallest element, and francium is the largest.
the chemical symbol for phosphorus is
A po
B p
C pt
D k
Answers
Answer:
B
Explanation:
Phosphorus is a chemical element with the symbol P and atomic number 15.
Answer:
The chemical symbol for phosphorus is letter A:po
Explanation:
Hope it help
Iodine-123, which is used for diagnostic imaging in the thyroid, has a half life of 13hrs. If 50. 0 mg of iodine 123 were prepared at 8am on monday, how many mg remain at 10 am on the following day?
Answers
Remaining amount ≈ 48.38 mg
Approximately 48.38 mg of iodine-123 will remain at 10 am the following day.
To determine the amount of iodine-123 remaining at 10 am the following day, we need to calculate the number of half-lives that have passed from 8 am on Monday to 10 am the next day.
Since the half-life of iodine-123 is 13 hours, there are (10 am - 8 am) / 13 hours = 2 / 13 = 0.1538 of a half-life between those times.
Each half-life reduces the amount of iodine-123 by half. Therefore, the remaining amount can be calculated as:
Remaining amount = Initial amount * (1/2)^(number of half-lives)
Initial amount = 50.0 mg
Number of half-lives = 0.1538
Remaining amount = 50.0 mg * (1/2)^(0.1538)
Remaining amount ≈ 50.0 mg * 0.9676
Remaining amount ≈ 48.38 mg
Approximately 48.38 mg of iodine-123 will remain at 10 am the following day.
Learn more about Remaining amount here
https://brainly.com/question/11991843
#SPJ11
which of the following is not true about rate laws? select one or more: rate laws are experimentally determined to relate the rate of the reaction to the concentrations. if the activation energy is small, the rate constant is also small, and the reaction is slower. larger values of k, the rate constant, indicate faster reaction rates. a catalyst can lower the activation energy of a reaction by providing an alternate path between reactants and products. since the rate constant is dependent on temperature, temperature must be recorded and held constant in order to study other kinetic aspects of the reaction the frequency factor, a, indicates how often a collision of reactants leads to the desired product. the arrhenius equation gives the relationship between the rate constant and the thermodynamics of the reaction. the activation energy, ea, is the energy required to start the reactions. high temperature correlates to a larger rate constant and a faster reaction.
Answers
The statement that is not true about rate laws is: "if the activation energy is small, the rate constant is also small, and the reaction is slower." Option b is correct.
In reality, if the activation energy is small, the rate constant is larger, and the reaction is faster. This is because a smaller activation energy means that more molecules have enough energy to react, so there are more effective collisions between reactants.
The other statements in the options are true about rate laws, including the fact that they are experimentally determined, that larger values of k indicate faster reaction rates, and that temperature affects the rate constant. Therefore, Option b is correct.
To know more about the rate laws, here
brainly.com/question/30379408
#SPJ4
--The complete question is, Which of the following is not true about rate laws?
a. rate laws are experimentally determined to relate the rate of the reaction to the concentrations.
b. if the activation energy is small, the rate constant is also small, and the reaction is slower. c. larger values of k, the rate constant, indicate faster reaction rates. a catalyst can lower the activation energy of a reaction by providing an alternate path between reactants and products.
c. since the rate constant is dependent on temperature, temperature must be recorded and held constant in order to study other kinetic aspects of the reaction the frequency factor, a, indicates how often a collision of reactants leads to the desired product. the arrhenius equation gives the relationship between the rate constant and the thermodynamics of the reaction.
d. the activation energy, ea, is the energy required to start the reactions. high temperature correlates to a larger rate constant and a faster reaction.--
The types of emission that mostly occur when an isotope undergoes radioactive decay include.
Answers
The types of emissions that mostly occur when an isotope undergoes radioactive decay include alpha particles, beta particles, and gamma rays.
The types of emissions that can occur when an isotope undergoes radioactive decay are alpha, beta, and gamma radiation. Alpha particles are made up of two protons and two neutrons and are positively charged. Beta particles are either electrons or positrons, and they are negatively or positively charged, respectively. Gamma radiation is a high-energy electromagnetic radiation that has no charge.
During alpha decay, the nucleus of an atom emits an alpha particle. This changes the atomic number of the element, but not its mass number. Beta decay occurs when a neutron in the nucleus of an atom transforms into a proton and an electron or positron is emitted. This can change the atomic number of the element, but not its mass number. Gamma decay occurs when the nucleus of an atom releases energy in the form of gamma radiation. This does not change the atomic number or mass number of the element.
To know more about radioactive decay visit:-
https://brainly.com/question/1770619
#SPJ11
the magnetic force of a material comes from the spinning of what atomic particle?a) Protonsb) Electronsc) Neutronsd) Photons
Answers
The magnetic force of a material comes from the spinning of electrons.
Electrons have a property called spin, which generates a magnetic moment. When many electrons in a material have their spins aligned in the same direction, they create a magnetic field.)
The magnetic force of a material originates from the spinning (also known as the magnetic moment) of electrons. This is because electrons have both charge and angular momentum, which together create a magnetic dipole moment and contribute to the overall magnetic properties of a material.
The magnetic force of a material comes from the spinning of electrons. Electrons have a property called spin, which generates a magnetic moment. When many electrons in a material have their spins aligned in the same direction, they create a magnetic field.)
Learn more about Electron
brainly.com/question/1255220
#SPJ11
Describe the trends in properties of chlorides across period 3 .Their formula .Their state .Their volatility .Their structure .The ph of aqueous chloride solution
Answers
Answer:
See explanation
Explanation:
Period 3 elements include; Na, Mg, Al, Si, P,S,Cl and Ar
Across the period, the chlorides of the elements change from solid to gas. The chlorides of metals are solid while the chlorides of non metals are gaseous.
Also, the chlorides become more volatile across the period. The formulas of the chlorides change from MCl to MCl2 to MCl3 to MCl4, MCl5 and MCl6 respectively across the period where M is a period 3 element.
The pH of the solutions of chlorides of period 3 elements change from neutral to acidic across the period. The pH of the chlorides of metals are neutral while the chlorides of the nonmetals usually yield acid solutions.
in the reaction of acetic acid with aqueous lithium hydroxide what is the spectator ion
Answers
In the reaction of acetic acid with aqueous lithium hydroxide, the spectator ion is lithium ion (Li+).
The balanced chemical equation for this reaction is:
\(CH_3COOH + LiOH\) → \(CH_3COOLi + H_2O\)
Acetic acid \((CH_3COOH)\)reacts with aqueous lithium hydroxide (LiOH) to form lithium acetate \((CH_3COOLi)\) and water (H2O). In this reaction, the lithium ion (Li+) is a spectator ion, meaning that it does not participate in the reaction and remains unchanged on both sides of the equation. Therefore, the spectator ion in the reaction of acetic acid with aqueous lithium hydroxide is lithium ion (Li+).
Here you can learn more about lithium hydroxide https://brainly.com/question/2528161
#SPJ11
Based on the article, which best replaces the question mark in the diagram above?
Butterfly Blues question 1

Answers
The statement that can replace the question mark is that the mutations would change with each generation.
What is mutation?The term mutation has to do with the changes that occurs in the genes of organisms. We have to note that mutations are caused by an alteration of the normal sequence of the proteins in the genes. There are several factors that could lead to the mutation of a gene and one of the factors that could lead to the mutation of a gene is the fact that the larva has been exposed to radiation.
We have to note that when we expose the larva to radiation, there would be a change in the genes and it is possible that this change in the structure of the genes could be passed on from one generation to the next hence the possibility that the kind of mutation would vary or change in each generation.
Learn more about mutation:https://brainly.com/question/13923224
#SPJ1
The atmospheric pressure on a mountain is 550 mmHg and 1 atm is equal to 760mmHg. What is the pressure in atm? Give your answer to 2 significant figures atm
Answers
1 atm
550 mmHg x —————- =0.72 atm
760 mmHg
Describe how nucleic acid basis pair up
Answers
The rules of base pairing (or nucleotide pairing) are: A with T: the purine adenine (A) always pairs with the pyrimidine thymine (T) C with G: the pyrimidine cytosine (C) always pairs with the purine guanine (G)
The nucleotides in a base pair are complementary which means their shape allows them to bond together with hydrogen bonds. The A-T pair forms two hydrogen bonds. The C-G pair forms three. The hydrogen bonding between complementary bases holds the two strands of DNA together.
Use the conversion that 1 ounce is approximately 28. 35 grams to calculate the unit price per ounce for 283. 5 grams at $5. 48. A. $0. 06 b. $0. 55 c. $5. 48 d. $54. 80.
Answers
The unit price per ounce is $0.55. The correct option is b. $0. 55
From the question,
We are to calculate the unit price per ounce
First, we will calculate the how many ounces are in 283.5 grams
From the give information,
28.35 g = 1 ounce
∴ 283.5 g = \(\frac{283.5}{28.35}\) ounces
283.5g = 10 ounces
Now,
Since the unit price is 283. 5 grams at $5. 48
That means
The unit price is 10 ounces at $5. 48
Now,
If 10 ounces cost $5.48
Then,
1 ounce will cost \(\frac{\$5.48}{10}\)
1 ounce will cost $0.548 ≅ $0.55
Hence, the unit price per ounce is $0.55. The correct option is b. $0. 55
Learn more here: https://brainly.com/question/1821352
which of the following was not a ""lesson"" that the egyptians learned from the hyksos invasion?
Answers
Based on the analysis, the lesson that the Egyptians did not learn from the Hyksos invasion is option e) The reliance on isolationism.
To identify the lesson that the Egyptians did not learn from the Hyksos invasion, we need to understand the historical context of the event and the subsequent actions taken by the Egyptians. The Hyksos invasion occurred during the Second Intermediate Period of ancient Egypt (17th century BCE) when a Semitic-speaking people from the Levant conquered Lower Egypt.
Step 1: Identify the lessons learned from the Hyksos invasion:
a) The importance of military strength: The Egyptians learned the significance of a powerful military to protect their borders and maintain stability.
b) The adoption of new military technologies: The Hyksos introduced horse-drawn chariots, composite bows, and other military advancements. The Egyptians learned the value of incorporating such technologies into their own military.
Step 2: Analyze the options:
c) The importance of diplomacy and alliances: The Hyksos invasion highlighted the need for Egypt to forge alliances with other regional powers. This lesson was likely learned by the Egyptians.
d) The significance of cultural assimilation: The Hyksos introduced aspects of their own culture to Egypt, including the worship of foreign deities. The Egyptians likely learned the importance of cultural assimilation to prevent social unrest.
Step 3: Determine the answer:
Based on the analysis, the lesson that the Egyptians did not learn from the Hyksos invasion is option e) The reliance on isolationism. The Egyptians recognized the importance of engaging with the outside world, forming alliances, and adopting new military technologies. Isolationism would have hindered their ability to defend against future invasions and integrate beneficial influences from other cultures.
Learn more about Hyksos invasion from this link:
https://brainly.com/question/2496881
#SPJ11
The option that was not a ""lesson"" that the Egyptians learned from the hyksos invasion is X bronze metallurgy the best defense
What was the Hyksos invasion?According to legend, a mystery tribe of alien invaders known as the Hyksos took control of the Nile Delta around 1638 B.C.However, there are few documented accounts of the dynasty, and modern archaeologists have uncovered little physical remnants of the historic military operation.
An invasion is when an army enters a territory, typically as part of a hostile attack during a war or other conflict. The world's history is replete with accounts of invasions.
Learn more about invasion at:
https://brainly.com/question/13882416
#SPJ4
missing options;
Security depended upon the maintenance of ma'at.
X bronze metallurgy the best defense
Type the correct answer in the box. use numerals instead of words. a certain radioactive atom has 90 protons and 142 neutrons. if this atom undergoes alpha decay, what is the mass number of the daughter atom? the mass number of the daughter atom is .
Answers
The mass number of the daughter atom formed from the alpha decay of a certain radioactive atom that has 90 protons and 142 neutrons is 228.
What is alpha decay?Alpha decay of a radioactive material is the release of an alpha particle that posseses mass number of 4 and atomic number of 2.
According to this question, a certain radioactive atom that has 90 protons and 142 neutron undergoes alpha decay. This means that the mass number of the daughter atom will be as follows:
Mass number of radioactive atom = 142 + 90 = 232
Mass number of daughter atom = 232 - 4 = 228
Therefore, the mass number of the daughter atom formed from the alpha decay of a certain radioactive atom that has 90 protons and 142 neutrons is 228.
Learn more about mass number at: https://brainly.com/question/4408975
#SPJ4
3. A cylinder compresses 50ml of air down to 10ml., if the temperature does not change what is
the pressure of air at the smaller volume?
Answers
The gas is compressed, the pressure of the gas increases. In this case, the cylinder compresses 50ml of air down to 10ml without any change in temperature. This means that the volume of the gas has decreased, which would cause an increase in pressure.
The calculate the pressure of the air at the smaller volume, we can use the formula P1 x V1 = P2 x V2 Where P1 is the initial pressure, V1 is the initial volume, P2 is the final pressure, and V2 is the final volume. We know that the initial volume is 50ml and the final volume is 10ml. We also know that there is no change in temperature, so the initial pressure and final pressure are equal. Therefore, we can rewrite the formula as P1 x 50ml = P1 x 10ml Solving for P1, we get P1 = (10ml/50ml) x P1 = 0.2 x P1 This means that the pressure of the air at the smaller volume is 0.2 times the initial pressure. If we assume that the initial pressure was atmospheric pressure (around 101 kPa), then the final pressure would be P2 = 0.2 x 101 kPa P2 = 20.2 kPa Therefore, the pressure of the air at the smaller volume is approximately 20.2 kPa.
learn more about pressure here.
https://brainly.com/question/22480179
#SPJ11
how many half lives would it take for 6.02 x10^23 nuclei to decay at 6.25% (0.376x10^23) of the original number of nuclei
Answers
To determine the number of half-lives required for a given percentage of nuclei to decay, the formula is:
\(N_f=N_i*(\frac{1}{2} )^n\)
where Nf is the final number of nuclei, Ni is the initial number of nuclei, and n is the number of half-lives.
Given information,
Ni = 6.02 x 10²³ nuclei
Nf = 0.376 x 10²³ nuclei
Thus,
0.376 x 10²³ = (6.02 x 10²³) × (1/2)^(n)
Dividing both sides of the equation by (6.02 x 10²³):
0.376 / 6.02 = (1/2)^(n)
0.0625 = (1/2)^(n)
Take the logarithm of both sides using a base of 1/2:
log base (1/2) of 0.0625 = n
log base 2 of 0.0625 / log base 2 of (1/2) = n
(-4) / (-1) = n
4 = n
Therefore, it would take 4 half-lives for 6.02 x 10²³ nuclei to decay to 0.376 x 10²³ nuclei.
Learn more about half-life, here:
https://brainly.com/question/31666695
#SPJ1
Based upon the aggie honor system rules and the academic integrity statement on the chem 111/112/117 syllabus, assess whether the statements are true or false.
Answers
TRUE - Asking for and receiving a paper that someone who is in another lab section wrote is an honor violation.
FALSE - An oral discussion with a classmate regarding a paper's topics and format is cheating.
FALSE - Purchasing papers from tutoring companies is allowed. False
TRUE - Sending a paper that you wrote to a student who is currently in another section is an honor violation.
TRUE - Misconduct in research or scholarship includes fabrication, falsification, or plagiarism in proposing, performing, reviewing, or reporting research. It does not include honest error or honest differences in interpretations or judgements of data.
TRUE - Discussing an exam or laboratory practical with anyone prior to the conclusion of the week that final exams and laboratory practicals are given would be considered an honor violation.
The Texas A&M University has an academic integrity statement known as the "Aggie code of honor." It outlines the rules for ethical academic conduct and responsible research. All curricula must include this code, according to instructors. It prohibits misconducts like plagiarism, falsification, and cheating and focuses on the use of the highest level of integrity in academic research.
Here is another question with an answer similar to this about academic integrity statement: https://brainly.com/question/14997968
#SPJ4
Question correction:
Based upon the Aggie Honor System Rules and the Academic Integrity statement on the CHEM 111/112/117 syllabus, assess whether the statements are true or false.
Asking for and receiving a paper that someone who is in another lab section wrote is an honor violation.An oral discussion with a classmate regarding a paper's topics and format is cheating.Purchasing papers from tutoring companies is allowed.Sending a paper that you wrote to a student who is currently in another section is an honor violation.Misconduct in research or scholarship includes fabrication, falsification, or plagiarism in proposing, performing, reviewing, or reporting research. It does not include honest error or honest differences in interpretations or judgements of data.Discussing an exam or laboratory practical with anyone prior to the conclusion of the week that final exams and laboratory practicals are given would be considered an honor violation.how fast will benzene solidify
Answers
Answer:
Very fast
Explanation: