Identify the waste gas removed from the blood during expiration.
A. Oxygen
B. Carbon Dioxide
C. Water
D. All of the above
Answers
the answer is carbon dioxide option b
please mark me the brainliest
Related Questions
What is the pressure in atm of 0.47mol of a gas in a 1.7L container at 276K?
Answers
2. A sample of gas is placed in a container at 25oC and 2 atm of pressure. If the temperature is raised to 50oC, what is the new pressure? P = 2.17 atm
3. At 1 atm of pressure water boils at 100oC, if the sample was placed under 2 atm of pressure, what would be the temperature? (This would be like a pressure cooker).
T = 746 K = 473oC = 883oF
4. At what temperature would water boil if the pressure is 600 torr? (Use information from problem 3: this shows why food doesn't cook well at higher elevations)
T = 294 K = 21.5oC = 70.7oF
5. Calculate the volume of 40.6 g of F2 at STP. V = 23.9 L
6. A sample of 2.0 moles of hydrogen gas is placed in a container with a volume of 10.4 L. What is the pressure of the gas in torr if the gas is at 25oC? P = 4.70 atm = 3576
7. The tire pressure is 32 psi. What is the pressure in torr if 1 atm = 14.7 psi?
P = 1654 torr
8. A gas is placed in a balloon with a volume of 3.0 L at 28oC and 900 torr. What would be the new volume for the gas if placed under STP? V = 3.2 L
9. How many moles of gas would occupy a volume of 14 L at a pressure of 700 torr and a temperature of 30oC? n = 0.52 mol
10. Calculate the volume of 24.0 g of HCl at STP. V = 14.8 L
11. What is the volume of one mole of acetylene gas at STP? V =22.414 L
12. What is the volume of 0.75 mol of gas at 72oC and 2 atm? V = 10.6 L
13. After eating beans, a student collects a sample of gas at 0.97 atm and 26oC which occupies a volume of 3.5 L, calculate its volume at STP. V = 3.1 L
14. Ammonia (NH3) is placed in 1.5 L flask at 25oC. If the pressure of the gas is 0.899 atm, what is the density? d = 0.626 g/L
15. A mixture of Ar and CO gases is collected over water at 28oC and an atmospheric pressure of 1.05 atm. If the partial pressure of Ar is 600 torr, what is the partial pressure of CO? (vapor pressure of water at 28oC is 28.3 mmHg) PCO = 0.223 atm
16. Determine the partial pressures of each of the gases in the following mixture: 17.04 g NH3, 40.36 g Ne and 19.00 g F2. The gases are at 1.5 atm of pressure.
PNH3 = 0.428 atm; PNe = 0.857 atm; PF2 = 0.2124 atm
17. Potassium chlorate decomposes under heat as follows:
2 KClO3 (s) -------> 2 KCl (s) + 3 O2 (g)
The oxygen gas is collected over water at 25oC. The volume of gas is 560 mL measured at 1 atm. Calculate the number of grams of KClO3 used in the reaction. (vapor pressure of water = 0.0313 atm) nO2 = 0.022 mol; 1.81 g KClO3
The nucleus contains protons and neutrons true or false?
Answers
Answer:
the answer is
it's true
Answer:
true
Explanation:
it has both neutrons (neutral) and protons (positive)
Hurry need the answer asap

Answers
P,T,Y refers to the set of points which are collinear and is therefore denoted as option C.
What are Collinear points?This is commonly used in geometry and refers to the set of points which lie on the same straight line and are always close to each other when compared to other points. It is possible for them to appear on different planes only and the value by the triangle from the three points is usually zero.
In the example give, only P,T and Y are the points which lie on the same line while other options have points which lie on different lines thereby making them incorrect.
This is therefore the reason why option C was chosen as the most appropriate choice.
Read more about Collinear points here https://brainly.com/question/18559402
#SPJ1
Fill The Blank? an ___________________ is the electrode where electrons flow out of a device.
Answers
An cathode is the electrode where the electrons flow out of the device.
The cathode is the type of the electrode where the electricity is given out or will flows out. The cathode is the negative side. The cathode is the negative as electrical energy which is supplied to the cell and it results in the decomposition of the chemical compounds.
The cathode can also be the positive as in the case of the galvanic cell where the chemical reaction will leads to the generation of the electrical energy. The cathode can be said to be either the hot cathode or the cold cathode.
To learn more about electrodes here
https://brainly.com/question/20090746
#SPJ4
Using standard heats of formation, calculate the standard enthalpy change for the following reactions.
a. Fe3O4(s)+4H2(g)→3Fe(s)+4H2O(g)
b. C(s,graphite)+O2(g)→CO2(g)
c. NH2Cl(aq)→NH3(g)+HCl(aq)
Answers
The reaction between CH4(g) + 2O2(g) and CO2(g) + 2H2O(l) results in an enthalpy change of -891 kJ/mol.
When given the temperatures of formation, how can we determine the standard enthalpy change?The standard enthalpy change of formation, in this equation, is defined as the product of the standard enthalpies of formation of the reactants and the sum of the standard enthalpies of formation of the products. Along with the data for the normal formation enthalpy: H fo[A]=433 KJ/mol. H fo[B] = -256 KJ/mol. The reaction enthalpy can be calculated by subtracting the sum of the enthalpies of all the reactants from the sum of the enthalpies of the products. By subtracting the sum of the enthalpies of the reactants from the total enthalpies of the product, we may get the thermodynamic constant, or tH.To learn more about Enthalpy change refer to:
https://brainly.com/question/16387742
#SPJ4
Cu(s) + 4 HNO3 (aq) --> Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O(l)
Each student in a class placed a 2.00g sample of a mixture of Cu and Al in a beaker and placed the beaker in a fume hood. The students slowly poured 15.0mL of 15.8M HNO3(aq) into their beakers. The reaction between the copper in the mixture and the HNO3(aq) is represented by the equation above. The students observed that a brown gas was released from the beakers and that the solutions turned blue, indicating the formation of Cu2 (aq). The solutions were then diluted with distilled water to known volumes. Which of the following is true about the reaction?
A)16%.
B) 32%.
C) 64%.
D) 96%.
Answers
The percentage of copper (Cu) in the original mixture, calculated from the given data, is 188%. None of the given options (A, B, C, or D) accurately represent the calculated percentage.
To determine the percentage of copper (Cu) in the original mixture, we can use stoichiometry and the concept of limiting reactants.
From the balanced chemical equation:
1 mole of Cu reacts with 4 moles of HNO3 to form 1 mole of Cu(NO3)2.
Given that the students added 15.0 mL of 15.8 M HNO3, we can calculate the number of moles of HNO3 added:
moles of HNO3 = (15.0 mL) * (0.0158 mol/mL) = 0.237 mol HNO3
Since the stoichiometric ratio between Cu and HNO3 is 1:4, we need four times the moles of HNO3 for a complete reaction with Cu. Therefore, the number of moles of Cu in the original mixture can be calculated as:
moles of Cu = 0.237 mol HNO3 * (1 mol Cu / 4 mol HNO3) = 0.05925 mol Cu
Now we can calculate the mass of Cu in the original mixture:
mass of Cu = moles of Cu * molar mass of Cu
mass of Cu = 0.05925 mol * 63.55 g/mol = 3.76 g Cu
Finally, we can calculate the percentage of Cu in the original mixture:
percentage of Cu = (mass of Cu / total mass of the mixture) * 100
percentage of Cu = (3.76 g / 2.00 g) * 100 = 188%
To read more about copper, visit:
https://brainly.com/question/26449005
#SPJ11
What values are needed to determine the energy of an electron?.
Answers
It determines the energy level of the electron. As n increases, the energy of the electron also increases. There is a maximum of seven energy levels, corresponding to values of n ranging from 1 to 7.
Azimuthal quantum number (l):
It defines the shape of the electron's orbital and its angular momentum. The value of l is dependent on the value of n and can range from 0 to n-1. Magnetic quantum number (m):
It identifies the specific orbital in which the electron is present and the direction of the electron's spin.
Electronic spin quantum number (s): It specifies the orientation of the electron's spin. The value of s can be either\(+1/2 or -1/2\). The values of these quantum numbers are used to calculate the total energy of an electron using the formula\(E = -13.6 eV/n².\)
To know more about electron visit:
https://brainly.com/question/12001116
#SPJ11
3. What are the values of the graduations (urmarked linas) in each of the
following graduated cylinders?
Answers
Answer:pp
Explanation:
When determining whether a chemical reaction has taken place, you observe and look for several indicators. Which would be considered an indication that a chemical reaction or chemical change has taken place?.
Answers
For getting an indication whether a chemical reaction or chemical change has taken place, if B)Heat is given off then chemical reactions occur. So,correct option is B.
Chemical reactions are surrounding us, from the digestion of food in our body to how the light we get from the sun is the consequence of compound responses. Prior to starting with compound responses, realizing about physical and synthetic changes is significant
Option A can't be the response is dissolving is an actual change. No substance response occurred.
Option B is the response as it is an EXOTHERMIC reaction so intensity will be emitted.
Option C can't be the response as dissolving is essentially ions becoming particles, not a synthetic response by which a reactant responds with one more reactant to shape an item.
Option D can't be the response. Same explanation with respect to why An isn't the response.
Hence, correct option is B.
To know more about chemical reactions, visit here:
https://brainly.com/question/29762834
#SPJ4
(Complete question) is:
When determining whether a chemical reaction has taken place, you observe and look for several indicators. Which would be considered an indication that a chemical reaction or chemical change has taken place? A) Solid melts. B) Heat is given off. C) Substance dissolves D) Substance changes shape.
Which statement is FALSE?
Mass number - Atomic Number = Neutrons
Mass number = protons + neutrons
Atomic number is the number of protons
O The periodic table is arranged by atomic mass
Answers
Answer:Mass number - Atomic Number = Neutrons
Explanation:
From the viewpoint of the chemical reactants as the system, what do you expect for the signs of q and w in this process?.
Answers
When q and w are positive, energy flows into the system.
When atoms establish or break chemical bonds, chemical processes take place. Reactants are the substances that begin a chemical reaction, while products are the compounds that are created as a result of the reaction.
What are reactants and products in chemistry?Summary. A chemical reaction is described by an equation in chemistry. The left side of the equation lists reactants as the initial materials. The right-hand side of the equation lists the products, which represent the outcome of the reaction.In a chemical reaction, reactants undergo a chemical reaction and transform into products through a chemical process. For instance, when humans breathe in oxygen, it combines with glucose to create carbon dioxide, water, and energy. The response is provided below. C6H12O6 + O2 = 6CO2 + 6H2O + Energy.When atoms establish or break chemical bonds, chemical processes take place. Reactants are the substances that begin a chemical reaction, while products are the compounds that are created as a result of the reaction.To learn more about Chemical reaction refer to:
https://brainly.com/question/11231920
#SPJ4
What is the acceleration of a ball traveling horizontally with an initial velocity of 20 meters/seconds and, 2.0 seconds later, a velocity of 30 meters/seconds?
Answers
acceleration = (change in velocity) / time
In this case, the change in velocity is:
30 meters/second - 20 meters/second = 10 meters/second
The time interval is:
2.0 seconds
So, the acceleration is:
10 meters/second / 2.0 seconds = 5 meters/second^2
Therefore, the acceleration of the ball is 5 meters/second^2.
Time Evolution in a Three-State System [15 points] Carbon dioxide is a linear molecule (OCO) that can pick up an extra electron and become a negatively charged ion. Suppose that the electron would have energy EO if it were attached to either oxygen atom, or energy EC if it were attached to the carbon atom in the middle. Call these states ∣L⟩,∣C⟩ and ∣R⟩, for left oxygen, carbon, and right oxygen. The energy eigenstates need not, however, have either energy EO or EC because there is some probability that the electron may hop between an oxygen atom and the carbon atom. (Assume that the probability of jumping directly from oxygen to oxygen can be neglected.)
Answers
The specific values of EL, EC, and ER will determine the precise behavior of the system and the probabilities of the electron being in each state over time.
To study the time evolution in this three-state system, we can write down the Hamiltonian matrix for the system.
The Hamiltonian describes the energy of the system and the transitions between different states. In this case, we have three states ∣L⟩, ∣C⟩, and ∣R⟩.
The Hamiltonian matrix can be written as:
H = [[EL 0 EC],
[0 EC 0],
[EC 0 ER]]
Here, EL represents the energy of state ∣L⟩, EC represents the energy of state ∣C⟩, and ER represents the energy of state ∣R⟩.
Since the electron can hop between the carbon atom and the oxygen atoms, we have nonzero off-diagonal elements in the Hamiltonian.
To solve for the time evolution of the system, we can find the eigenvalues and eigenvectors of the Hamiltonian matrix.
The eigenvectors represent the energy eigenstates of the system, and the eigenvalues represent the corresponding energies.
Once we have the energy eigenstates, we can determine the probabilities of the electron being in each state at a given time by calculating the time evolution of the initial state.
The specific values of EL, EC, and ER will determine the precise behavior of the system and the probabilities of the electron being in each state over time.
It is worth noting that the problem statement doesn't provide explicit values for EL, EC, and ER, so further information is needed to obtain a more detailed analysis of the time evolution in this three-state system.
Learn more about Hamiltonian matrix from this link:
https://brainly.com/question/29556622
#SPJ11
How many different signals would you see in the carbon NMR?
Answers
It is difficult to determine the exact number of signals that would be seen in the carbon-13 (¹³C) nuclear magnetic resonance (NMR) spectrum without knowing the specific compound being analyzed.
In general, the number of signals in the ¹³C NMR spectrum is determined by the number of chemically distinct carbon environments in the molecule. Each type of carbon atom in a molecule has a different chemical shift value, which depends on the electronic environment and neighbouring atoms. Therefore, the number of signals seen in the ¹³C NMR spectrum is equal to the number of unique carbon environments in the molecule.
For example, a simple organic molecule such as ethanol (CH₃CH₂OH) has two unique carbon environments: the methyl carbon (CH₃) and the methylene carbon (CH₂). Thus, the ¹³C NMR spectrum of ethanol would show two signals.
More complex molecules may have many different carbon environments, leading to a more complex ¹³C NMR spectrum with many signals.
To learn more about atoms Click here:
brainly.com/question/1566330
#SPJ4
could the z-isomer of 1,2-bis(4-methoxyphenyl)ethene be distinguished from the e-isomer based on their 1h nmr spectra in the same manner as question 2 above? explain.
Answers
Yes, the z-isomer and e-isomer of 1,2-bis(4-methoxyphenyl)ethene can be distinguished based on their 1H NMR spectra. The 1H NMR spectra of the two isomers would show different chemical shifts for the protons in the vicinity of the double bond due to the different orientations of the two phenyl groups.
The z-isomer would have a higher chemical shift due to the cis-configuration of the two phenyl groups, while the e-isomer would have a lower chemical shift due to the trans-configuration of the two phenyl groups. Additionally, the coupling constants between the protons in the vicinity of the double bond would be different for the two isomers, with the z-isomer having a larger coupling constant due to the cis-configuration and the e-isomer having a smaller coupling constant due to the trans-configuration.
Therefore, the 1H NMR spectra can be used to distinguish between the two isomers of 1,2-bis(4-methoxyphenyl)ethene.
It seems that I don't have access to the information from question 2 you mentioned. However, I can still help you understand if the Z-isomer of 1,2-bis(4-methoxyphenyl)ethene can be distinguished from the E-isomer based on their 1H NMR spectra.Yes, the Z-isomer and E-isomer of 1,2-bis(4-methoxyphenyl)ethene can be distinguished based on their 1H NMR spectra. Here's a step-by-step explanation:
1. Understand that the Z-isomer and E-isomer are geometrical isomers due to the presence of a double bond between two carbon atoms in the ethene part of the molecule. The Z-isomer has the two 4-methoxyphenyl groups on the same side of the double bond, while the E-isomer has these groups on opposite sides.
2. Geometrical isomers can have different spatial arrangements, which can lead to differences in their chemical environments, especially for the hydrogen atoms adjacent to the double bond.
3. When analyzing the 1H NMR spectra, look for differences in the chemical shift values and the splitting patterns of the hydrogen atoms in both isomers. Since the chemical environments are different for the Z- and E-isomers, their 1H NMR spectra will show differences in these parameters.
4. The differences in chemical shift values and splitting patterns in the 1H NMR spectra can be used to distinguish between the Z- and E-isomers of 1,2-bis(4-methoxyphenyl)ethene.
By comparing the 1H NMR spectra of the Z- and E-isomers, you can identify which isomer you have based on the unique spectral signatures each isomer presents.
Learn more about NMR here:- brainly.com/question/29885193
#SPJ11
A molecule that organisms get from the air or water around them is
Answers
What is the pOH of a solution with a pH of 8.99?
Answers
Answer:
First To find the pOH, simply subtract the pH from 14. In order to calculate the pOH, take the negative log of the hydroxide ion concentration. To find the pH, simply subtract pOH from 14.
The given $pOH $ of solution is 8.99. To find its pH value we will use the formula, $pOH=14-pH$. So, pH = 14-8.99 = 5.01.
Explanation:
Of the following, which can be used to identify an element present in a sample?
Answers
Answer:
idk but i tryed
Explanation:
The simplest way to use the periodic table to identify an element is by looking for the element’s name or elemental symbol. The periodic table can be used to identify an element by looking for the element’s atomic number. The atomic number of an element is the number of protons found within the atoms of that element.
Constituent elements of bronze
Answers
Answer:
Bronze is an alloy.
Explanation:
Bronze is an alloy consisting primarily of copper, commonly with about 12 to 12.5%tin and often with the addition of other metal such as aluminium, nickel, manganese and zinc.
what advice would you give to your friend who has been suffering from domestic violence
Answers
Answer:
the advices are,
1. i would say him or her to give all information about not to do domestic violence who has doing it.
2. i would say him/ herto be strict towards the domestic violence.
3. i would suggest him or her to raise voice aganist towards the domestic violence.
5. i would suggest him or her to negotiate to them for theirbasic rights.
6. if its not possible then i would suggest him or her to complain to the police.
hope u like it ...
Answer: If my friend has been suffering from domestic violence, then I will advice her to be strict towards domestic violence, believe in yourself, don't lose hope, provide the information about not doing domestic violence for who has doing it, I will suggest her to raise voice against it, to fight for the basic rights, If not possible then i would advice her to complain to police and if it is even worst then I will advice her to leave as soon as possible, make your own decision, etc These advice i would give if my friend has been suffering from domestic violence.
Explanation:
Hope it will help you...................
Which term describes the phase change that occurs when intermolecular forces (attractions) completely overcome kinetic energy (motion) and the material has little to no motion?
Answers
Explanation:
If the kinetic energy is less than the attractive forces, a liquid or solid will form.
The average kinetic energy of the particles in a solid is very less almost negligible this is because they have high attractive forces between their molecules as they are tightly packed in regular lattices and are vibrating about their mean positions.
Therefore, freezing of a liquid or the sublimation of a gas results in the formation of a solid. These two phase changes result in the overcoming of K.E completely.
Answer:
freezing
Explanation:
The system below was at equilibrium in a
9.0 L container. What change will occur
for the system when the container is
shrunk to 3.0 L?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
Hint: How many moles of gas are on each side?
A. The reactions shifts to the right (products) to produce
fewer moles of gas.
B. There is no change because there are the same
number of moles of gas on both sides.
C. The reactions shifts to the left (reactants) to produce
more moles of gas.

Answers
The number of moles of gas is the same on both sides, the change in volume will not affect the equilibrium position of the reaction. The answer is B) There is no change because there are the same number of moles of gas on both sides.
To determine the change that will occur when the container is shrunk from 9.0 L to 3.0 L for the given reaction:
51.8 kJ + H₂(g) + I₂(g) → 2HI(g)
We need to consider the number of moles of gas on each side of the reaction.
On the left side, there are 2 moles of gas (H₂ and I₂), while on the right side, there are 2 moles of gas (2HI). Both sides have an equal number of moles of gas.
Therefore, the correct answer is B) There is no change because there are the same number of moles of gas on both sides.
For more details regarding the number of moles, visit:
https://brainly.com/question/20370047
#SPJ1
explain why vehicles of mombasa rust faster than vehicles at nairobi
Answers
Answer:
The primary reason why cars in Mombasa rust faster than those in Nairobi is because the humidity ( water evaporation rate) in Mombasa is higher than that of Nairobi. Since Mombasa has higher temperatures the rate of water evaporation is higher which results into a higer humidity.
Explanation:
This is why cars in Mombasa rust faster than those in Nairobi
NEED HELP ASAP PLEASE ANSWER!!!!! WILL MARK BRAINLEST!!!

Answers
4) ₆¹¹ C → ₊ ⁰₁e +¹¹₅ B
The above equation shows positron decay
What is positron decay ?
A specific kind of beta particle (+) is the positron. Another representation of a positron is ⁰₁e
An electron neutrino's symbol is Vе
If the neutron-proton ratio is less than 1:1, or if there are too many protons, the majority of nuclei are unstable. To make up for the imbalance, they will decompose.
The nucleus becomes more stable as a result of positron emission, which raises the proportion of neutrons and lowers that of protons. In positron emission, the mass number A stays constant while the atomic number Z drops by one.
To know more about positron click the link given below
brainly.com/question/21275312
#SPJ1
aluminum hydroxide + sodium nitrate reaction type
Answers
Answer:
.
Explanation:
calculate the number of moles of nitrogen monoxide gas used to form 72.0 grams of water vapor.
Answers
4 moles of nitrogen monoxide gas used to form 72.0 grams of water vapor.
What is a mole ?A mole is the measurement unit which is used to correlate the molar mass with number of molecules present.
The equation for reaction of Nitrogen monoxide to form water vapor is given by
4NH₃ + 6NO ----> 5N₂ + 6H₂O
72 grams of water vapor means = 72/18 = 4 mole of water vapour
The mole fraction of NO to H₂O is 6 : 6
Therefore for 4 moles of water vapor formation , 4 moles of nitrogen monoxide will be required.
To know more about mole
https://brainly.com/question/26416088
#SPJ1
the incidence of allergies has increased in recent years due to all of the following except
Answers
The incidence of allergies has increased in recent years due to all of the following except for one factor.
The increase in allergies has been attributed to various factors such as changes in diet, environmental pollution, and overuse of antibiotics. However, one factor that has not been linked to the rise in allergies is vaccination. Numerous studies have shown that vaccines do not cause allergies and are not a contributing factor to the increase in allergic reactions. In fact, vaccines have been proven to prevent serious and potentially life-threatening diseases that can further weaken the immune system and increase the risk of allergies. Therefore, it is important to continue promoting the benefits of vaccination to protect public health.
learn more about allergies
https://brainly.com/question/28341049
#SPJ11
I just want u to check if it’s correct or not no need for explanations
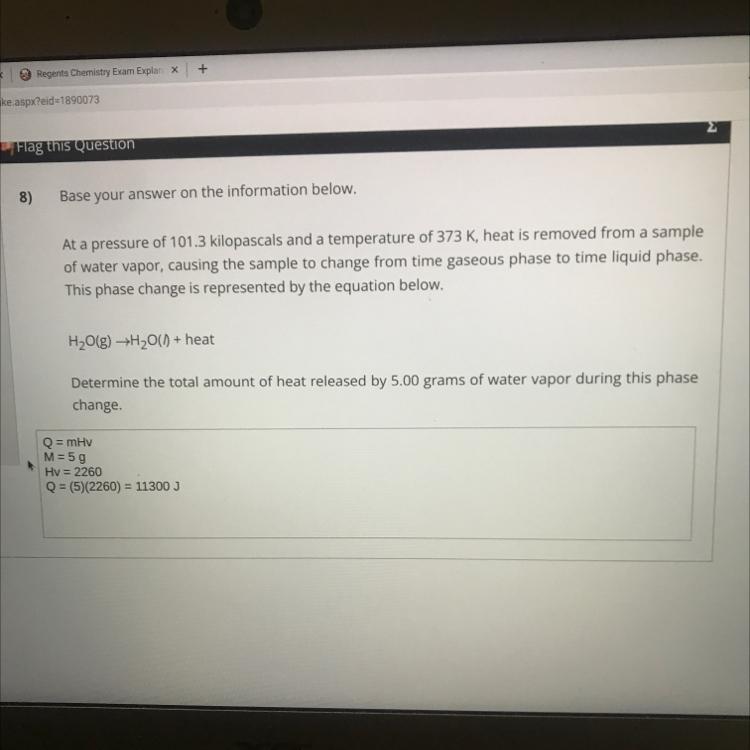
Answers
Given mass = 5g
Heat of vapour = 2260
Heat released during conversion of steam = m * C = 5 * 2260
= 11300J
Your calculations are correct.
what is the name of s and p blocks on the periodic table??
Answers
Answer:
The s-block and p-block together are usually considered main-group elements, the d-block corresponds to the transition metals, and the f-block encompasses nearly all of the lanthanides (like lanthanum) and the actinides (like actinium).
Explanation:
Two elements are found together in a substance. They are not chemically combined. Is this substance an element, a mixture or a compound?
Answers
substance