Answers
Answer:
This is the answer but I'm sure from all answers. I hope my answer help you.

Related Questions
What are the types of chemical bonds? Describe each.
Answers
Ionic bonds are formed when a metal and a nonmetal interact by exchange of electrons;
covalent bonds are formed when nonmetal atoms come together to share a few of their valence electrons;
metallic bonding happens when metals interact with one another.
When iron combines with oxygen gas and forms rust, the total mass of the products...
is the same as the mass of the reactants.
is less than the mass of the reactants.
depends on the reaction conditions.
is greater than the mass of the reactants.
Answers
Arrange the procedural steps, from start to finish, that are required for the correct use of an automatic micropipettor.
Answers
Steps that are required for the correct use of an automatic micropipettor -
1. ensure that the pipette is adjusted to discharge the proper V
2. depress the knob until you reach the point of first resistance.
3. dip the tip of the pipette into the solution
4. gradually depress the plunger to suck solution into the tip
5. Remove the tip from the solution and put it on the inside wall of the container into which the solution to be dispersed.
6. again depress the knob to the first point of resistance
7. After a brief pause, depress the knob as far as it will go.
What is the benefit of an automated pipette?Pipettes that are automated can eliminate the need for manual work and provide a variety of benefits. The most obvious benefit is increased throughput, since automation frees up time and effort for other activities. Another advantage is increased repeatability.
To learn more about micropippet from the given link
https://brainly.com/question/23793716
#SPJ4
what is the value of the dissociation constant, kd, for the complex ion zn(nh3)42 ? for zn(nh3)42 , kf
Answers
The value of the dissociation constant, kd, for the complex ion Zn(NH₃)₄²⁺ is 3.4 × 10⁻¹⁰.
the dissociation of Zn(NH₃)₄²⁺ is given as follows :
kd
Zn(NH₃)₄²⁺(qa) ⇄ Zn²⁺(aq) + 4NH₃(aq)
kf
the dissociation constant kd is given as :
kd = [Zn²⁺] [ NH₃]⁴ / [ Zn(NH₃)₄²⁺ ]
the kf is the reciprocal of the kd , that means :
kd = 1 / kf
the kf for Zn(NH₃)₄²⁺ is 2.9 × 10⁹
therefore :
kd = 1 / 2.9 × 10⁹
kd = 0.344 × 10⁻⁹
kd = 3.4 × 10⁻¹⁰.
Thus, the dissociation constant Zn(NH₃)₄²⁺ is 3.4 × 10⁻¹⁰.
To learn more about dissociation constant here
https://brainly.com/question/29588332
#SPJ4
can someone pls help

Answers
Answer:
Table D
Explanation:
First, remember the definitions of groups, periods, and valence electrons.
Groups are columns. On the periodic table they go from 1 to 18
Periods are rows. On the periodic table they range from 1 to 7
Valence electrons are electrons in the outermost shell of the atom in question. To determine the number of valence electrons, count which column (group) an atom is in from left to right. When counting which column an atom is in, do not count the transition metals (group 3-12) because these elements have variable valence electrons which do not follow this rule.
For example, since Ca is in group 2 (the second column from the left) this atom has two valence electrons.
Similarly, P has a valence electron number of 5 because we count from left to right: 1, 2, SKIP THE TRANSITION METALS (MIDDLE BLOCK), 3, 4, 5
Why do we calculate rms velocity?
Answers
The explanation we utilize the rms velocity rather than the normal is that for a commonplace gas test the typical speed is zero since the particles are moving every which way.
What is rms velocity?Root mean square (RMS) velocity is the square base of the amount of the squares of the stack speed values partitioned by the quantity of values. RMS speed is the speed of a wave going through underground developments at various span speeds along a given beam way.
For flat or marginally inclined layers, NMO and stacking speed are equivalent to RMS speed. In any case, in regions with huge parallel speed varieties, the stack velocity contrasts essentially from the RMS speed.
Conduction is the minuscule temperature inclination of the root-mean-square speed of issue. This substance can be either strong, fluid, or gas.
The explanation we utilize the rms velocity rather than the normal is that for a regular gas test the typical speed is zero since the particles are moving every which way.
To know more about rms velocity, visit:
brainly.com/question/29605566
#SPJ4
Determine whether each statement is a description of a physical property or a chemical property.
Paper burns readily in air.
physical
chemical
Salt is a solid.
physical
chemical
Several pieces of paper are burning in a grassy location.
Answers
Answer:
Paper=chemical
Salt=physical
Explanation:
Answer:
B.chemical
A.physical
Explanation:
0. 300 mole of urea (ch4n2o) in 2. 50 × 102 ml of solution
Answers
The molarity of the solution containing 0.300 moles of urea in 2.50 × 10^2 ml of solution is 1.20 M.
To calculate the molarity (M) of a solution, we use the formula:
Molarity (M) = moles of solute / volume of solution in liters
Given:
Moles of urea = 0.300 moles
Volume of solution = 2.50 × 10^2 ml = 250 ml = 250/1000 = 0.250 liters
Let's plug in the values into the formula:
Molarity (M) = 0.300 moles / 0.250 liters
Molarity (M) = 1.20 M
Therefore, the molarity of the solution containing 0.300 moles of urea in 2.50 × 10^2 ml of solution is 1.20 M.
Learn more about molarity from the link given below.
https://brainly.com/question/30908431
#SPJ4
what functional group would you expect from reaction of a primary amide with each of the following? if nothing occurs write no reaction. 1) lialh4, 2) h3o
Answers
1) The reaction of a primary amide with LiAlH₄ would result in the reduction of the amide functional group to a primary amine.
2) The reaction of a primary amide with H₃O⁺ would result in the hydrolysis of the amide functional group to form a carboxylic acid and ammonia.
1) LiAlH₄ is a strong reducing agent commonly used for the reduction of carbonyl compounds. In the presence of LiAlH₄, the primary amide undergoes reduction, where the carbonyl group (-C=O) is transformed into a primary amine (-NH₂), resulting in the removal of the oxygen atom.
2) H₃O⁺ represents an acidic environment and can initiate the hydrolysis of amides. In the presence of H₃O⁺, the amide functional group undergoes hydrolysis through a reaction called acid hydrolysis. This process cleaves the amide bond, breaking it into a carboxylic acid and an amine. The amine formed in this case would be ammonia (NH₃).
Overall, the reaction of a primary amide with LiAlH₄ results in the reduction to a primary amine, while the reaction with H₃O⁺ leads to the hydrolysis of the amide, forming a carboxylic acid and ammonia.
learn more about hydrolysis here:
https://brainly.com/question/31827573
#SPJ11
what is the atomic number of nickel
Answers
Answer:
28
Explanation:
How many molecules are contained in 2.34 moles of carbon monoxide?
Answers
Answer:
1.409 × 10²⁴ moleculesExplanation:
The number of carbon monoxide molecules can be found by using the formula
N = n × Lwhere n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
N = 2.34 × 6.02 × 10²³
We have the final answer as
1.409 × 10²⁴ moleculesHope this helps you
[H+] for a solution is 1 x 10^-7 M. This solution is _____________.
A. acidic
B. basic
C. neutral
Answers
Answer:
the answer is C neutral
Explanation:
if the ph is lower then 7 its acidic
if its higher then 7 its basic
and a ph of 7 is neutral
List 5 items and explain why these substances would be effective in cleaning a dirty penny.
Answers
Answer:
Alchohol, Disenfective wipes, Bleach, Soap,
Explanation:
Part of ecosystem Contains energy storage molecules? (yes or no) Energy storage molecules flowing in? (yes or no) Energy storage molecules flowing out? (yes or no)
Producers
yes
no
yes
Consumers
yes
yes
yes
Decomposers
yes
yes
yes
Dead matter
yes
yes
yes
Abiotic matter
Answers
Part of ecosystem | Contains energy storage molecules? (yes or no) | Energy storage molecules flowing in? (yes or no) | Energy storage molecules flowing out? (yes or no)
Producers | Yes | No | Yes
Consumers | Yes | Yes | Yes
Decomposers | Yes | Yes | Yes
Dead matter | Yes | Yes | Yes
Abiotic matter | No | No | No
In an ecosystem, different components play different roles in terms of energy storage and flow.
Producers, such as plants, have the ability to produce energy-rich molecules through photosynthesis, storing energy in the form of carbohydrates. They do not rely on external sources of energy storage molecules, but they release energy storage molecules into the ecosystem when consumed or when they undergo decomposition.
Consumers, including herbivores, carnivores, and omnivores, obtain energy storage molecules by consuming producers or other consumers. They receive energy-rich molecules flowing into their systems through their diet and release energy storage molecules when they respire or excrete waste.
Decomposers break down organic matter, including dead plants and animals, into simpler substances and release energy storage molecules in the process. They receive energy storage molecules flowing into their systems from the breakdown of organic matter and release energy storage molecules back into the ecosystem.
Dead matter, which refers to organic material that is no longer living, contains energy storage molecules. When dead matter decomposes, the stored energy is released into the ecosystem.
Abiotic matter, which includes non-living components like minerals and gases, does not contain energy storage molecules and does not participate in the flow of energy storage molecules within the ecosystem.
Learn more about ecosystem dynamics here:
https://brainly.com/question/31828234
#SPJ11
True or false: All molecules or atoms in a substance have the same amount of kinetic energy at a given temperature?
Answers
Answer:
1
False. if they all moved together at any time then it would be a million things withinthe human species all at once at a high rate
Penny bought a club moss plant for her water garden. She needs to know how tall the plant will grow so she knows how much space it will need.
How tall will the plant likely grow?
less than 5 centimeters because it is a seedless vascular plant
less than 5 centimeters because it is a nonvascular plant
more than 5 centimeters because it is a seedless vascular plant
more than 5 centimeters because it is a nonvascular plant
Answers
The plant will grow less than 5 centimeters because it is a seedless, non-vascular plant. The correct option is B.
What are moss plants?Moss plants are those plants that do contain seeds or flowering plants. They are non-vascular plants, and they maintain the moisture of the soils. They are very small plants.
Moss plants are small plants that grow in moist and humid conditions. They do not need so much sunlight. They will grow less than 5 centimeters and wet conditions.
Thus, the correct option is B. less than 5 centimeters because it is a non-vascular plant.
To learn more about moss plants, refer to the link:
https://brainly.com/question/3329215
#SPJ1
Summarize the process a scientist goes through to come up with a
satisfactory solution.
Answers
Select, from the following options, all that correctly describe the structural features which allow a species to exhibit resonance. Check all that apply. a. A double bond is adjacent to a positively charged carbon atom. b. Two adjacent atoms both have lone pairs of electrons. A C C bond is present in the structure. c. An atom with a lone pair is adjacent to an atom with a positive charge. d. A double bond is adjacent to an atom with a lone pair of electrons. Determine the monochlorination product(s)
Answers
The options given above correctly describe the structural features that allow a species to exhibit resonance.
A positively charged atom is next to an atom with a lone pair.Next to a positively charged carbon atom is a double bond.An atom with a single pair of electrons is next to another atom via a double bond.Next to an atom with a positive charge is an atom with a lone pair. A positively charged carbon atom is next to a double bond. An atom that has a single pair of electrons is next to a double bond.
Learn more about resonance here:
https://brainly.com/question/11331041
#SPJ4
PLS HELP I WILL GIVE BRAINLISTED AND I GIVE YOU SO MUCH STUFF FI NEED IT PLS

Answers
Photosynthesis removes CO2 and animal activities, burning fossil fuels and burning wood add CO2.
Why is CO2 needed?
The main greenhouse gas that helps keep our atmosphere warm is carbon dioxide. Without them, our earth would be uninhabitable. However, the rise in average global temperature due to rising levels of CO2 in our atmosphere is affecting other aspects of Earth's climate. Carbon dioxide is required for the internal respiration of the human body. Internal respiration is the process of removing carbon dioxide and delivering oxygen to body tissues.
The pH value of the blood is protected by the vital carbon dioxide. Therefore, only plants excrete CO2.
To learn more about Carbon dioxide, here
brainly.com/question/3049557
#SPJ1
For each of the formulas, classify the formula as either an empirical formula, a molecular formula, or both. Nahco3 c2h6.
Answers
Sodium bicaronate's empirical and molecular formulas are both NaHCO3. The empirical formula for C2 H6 is CH3.
What does "structural formula" mean?
Chemical bonds connecting the atoms of a molecule are located in structural formulae. A structural formula is made up of symbols for the atoms joined by brief lines that stand in for chemical bonds. Single, double, and triple bonds are denoted by one, two, or three lines, respectively.
If we look at the molecular formula for NaHCO3, we can see that it has a molar ratio of 1:1:1:3 and that everything in this molar ratio results in a total mass of: 23g + 1g + 12g + 48g = 84g. Knowing that the molecular formula shows the total molar ratio of each element in a compound, while the empirical formula shows the simplest ratio of the elements present in a compound.
To determine the empirical formula of C2 H6 , you divide the molecular formula by a number that gives the smallest ratio of atoms. So, we divide C2 H6 by 2 to determine the empirical formula CH3. Structural formula in picture given.
To learn more about structural formula use link belwo:
https://brainly.com/question/514499
#SPJ4
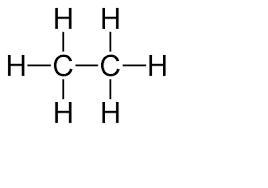
Answer:
Both and then Molecular
Explanation:
Please help How many moles of a gas sample are in 5.0 L container at 215 K and 342 kPa(The gas constant is 8.31 L kPa/mol K) Round your answer to one decimal place and enter the number only with no units.

Answers
Answer
1.0 mol
Explanation
Given:
Volume, V = 5.0 L
Temperature, T = 215 K
Pressure, P = 342 kPa
The gas constant, R = 8.31 L kPa/mol K
What to find:
The number of moles of the gas sample.
Step-step-solution:
The number of moles of the gas can be determine using the ideal gas equation formula:
\(PV=nRT\)Put the given values into the formula and calculate for n:
\(\begin{gathered} 342\times5.0=n\times8.31\times215 \\ 1710=1786.65n \\ \text{Divide both sides by 1786.65} \\ \frac{1710}{1786.65}=\frac{1786.65n}{1786.65} \\ n=0.9571\text{ mol} \\ To\text{ one decimal place,} \\ n=1.0\text{ mol} \end{gathered}\)The number of moles of the gas sample is 1.0 mol.
Are cyclops heterotrophic or autotrophic?
Answers
Cyclops are heterotrophic
what is FI5 compound name?
Answers
Answer:
this is the name for it PENTADECANOIC ACID
Explanation:
I kinda don't understand what you mean by the compound name but this is the scientific name, tell me if it helped :)
Calcula el volumen en litros que tendran 2 kg de poliestireno expandidos (densidad = 0,92g/cm3)
Answers
2 kg of expanded polyethylene has a volume of 2.17 liters.
Given that,
Density = 0.9g/cm³
Mass = 2kg = 2000g
Density is the substance's mass per unit of volume. Although the Roman letter D may also be used, the sign most frequently used for density is ρ (the lowercase Greek letter rho). A substance's density changes as a function of pressure and temperature. With solids and liquids, this variance is often slight, but for gases, it is much more pronounced.
Density = Mass ÷ Volume
0.92 = 2000 ÷ Volume
Volume = 2000 ÷ 0.92
Volume = 2.17 liters.
Hence, 2 kg of expanded polyethylene has a volume of 2.17 liters.
To learn more about density, refer to:
https://brainly.com/question/26364788
#SPJ4
Your question is in Spanish. The English translation of the question is:
Calculate the volume of 2 kg of expanded polyethylene in liters. ( Density = 0.92g/cm³ )
What is the number of nitrogen molecules that reacted with excess hydrogen to make 2 x 10^10 molecules of ammonia?
Answers
Stoichiometry and the mole concept state that a reaction between 1 x 1010 molecules and extra hydrogen produced 2 x 1010 molecules of ammonia.
What does stoichiometry mean?A chemical reaction's stoichiometry involves figuring out the ratios of its constituent elements or molecules. The underlying laws of the associated relationships are the law of mass preservation and the law on combining weights & volumes.
To determine the amounts of different compounds contained in the sample, stoichiometry is employed in quantitative analysis.
The balance of chemical equations requires it.
In the example provided, since 1 molecule of nitrogen creates 2 molecules of ammonia, 2 x 1010 molecules o ammonia will be created when 2 x 10101/2=1 molecule.
As a result, 1 x 1010 molecules formed 2 x 1010 molecules of ammonia when they were combined with extra hydrogen.
To know more about stoichiometry visit:
https://brainly.com/question/30215297
#SPJ4
Calculate the mass percent (m/m) of a solution prepared by dissolving 51.76 g of NaCl in 170.9 g of H2O . Express your answer to four significant figures.
Answers
The mass percent (m/m) of the solution prepared by dissolving 51.76 g of NaCl in 170.9 g of H2O is 23.25%.
To calculate the mass percent (m/m) of a solution, you need to divide the mass of the solute (NaCl) by the total mass of the solution (NaCl + H2O) and multiply by 100.
The mass of NaCl is given as 51.76 g, and the mass of H2O is given as 170.9 g.
To find the total mass of the solution, you simply add the masses of NaCl and H2O together: 51.76 g + 170.9 g = 222.66 g.
Next, divide the mass of NaCl by the total mass of the solution: (51.76 g / 222.66 g) * 100 = 23.25%.
To know more about mass percent please refer to:
https://brainly.com/question/26150306
#SPJ11
Element 1 2 3 4 5 Electronegativity 3.04 1.88 2.04 2.20 3.98 Which element would be most likely to identify as a metal
Answers
The element with the highest electronegativity value in the given list is 3.98, which corresponds to Element 5. Therefore, Element 5 would be the most likely to identify as a metal.
To know more about electronegativity and its relationship to metal identification, refer here:
Electronegativity is a measure of an element's ability to attract electrons in a chemical bond. Metals typically have lower electronegativity values compared to nonmetals. They tend to lose electrons easily to form positive ions and exhibit metallic properties such as conductivity, malleability, and luster.
In the given list, Element 5 has the highest electronegativity value of 3.98. This value is significantly higher than the other elements in the list. The large electronegativity suggests that Element 5 has a strong ability to attract electrons, which is characteristic of nonmetals rather than metals. Therefore, the remaining elements in the list (Elements 1, 2, 3, and 4) would be more likely to identify as metals.
In summary, Element 5, with an electronegativity value of 3.98, would be the least likely to identify as a metal among the given elements.
To know more about electronegativity, refer here:
https://brainly.com/question/3393418#
#SPJ11
a mixture of nitrogen and neon gas is expanded from a volume of 98 l to a volume of 100 l, while the pressure is held constant at . calculate the work done on the gas mixture. be sure your answer has the correct sign (positive or negative) and the correct number of significant digits.
Answers
Without methane, water vapor, and carbon dioxide gases in the atmosphere, Earth's surface would be frozen over. Group of answer choices True False
Answers
The statement "Without methane, water vapor, and carbon dioxide gases in the atmosphere, Earth's surface would be frozen over" is true because they trap heat from the sun in the Earth's atmosphere, creating the greenhouse effect.
This process helps to maintain a relatively stable temperature on Earth that is suitable for life. Without these greenhouse gases, the Earth's surface would not receive enough heat to counteract the cooling effects of radiation, and the temperature would drop below freezing, causing the planet's surface to be frozen over.
This scenario is known as a "snowball Earth" and has occurred in the distant past when there were significant changes in the atmospheric composition.
To know more about the atmosphere refer here :
https://brainly.com/question/26767532#
#SPJ11
PLEASE HELP ME! The question is on the image! It’s about the mole

