I need help with finding the Concentration of HCL (Ma)
the other things I have right now are
Vb = 10ml
Va = 10ml
Mb = 0.25m

Answers
Answer:
0.35 M
Explanation:
HCl + NaOH --> NaCl + H2O
Since the ratio HCl : NaOH is 1:1, you can apply the formula:
Ma x Va = Mb x Vb
Vb = 14 ml (based on the table)
Va = 10 ml
Mb = 0.25 M
Ma = (Mb x Vb)/Ma = (14 x 0.25)/ 10 = 0.35 M
Related Questions
What is the formula for Mn+2, Br
Answers
Answer:
MnBr₂
Explanation:
in the redox reaction 2bro3–(aq) 3n2h4(g) ⟶ 2br–(aq) 3n2(g) 6h2o(l) the oxidation number of bromine changes from _______ to _______.
Answers
In the redox reaction 2BrO3-(aq) + 3N₂H₄(g) ⟶ 2Br-(aq) + 3N₂(g) + 6H₂O(l), the oxidation number of bromine changes from +5 to -1.
Oxidation number is a number assigned to an atom in a chemical compound that represents the number of electrons lost (oxidation) or gained (reduction) by an atom when it forms a chemical bond with another atom.
In BrO₃-, the oxidation number of bromine is +5. This is because bromine is bonded to three oxygen atoms, each of which has an oxidation number of -2. The sum of the oxidation numbers of all the atoms in a molecule must be zero, so the oxidation number of bromine must be +5 to balance the negative oxidation numbers of the oxygen atoms.
In Br-, the oxidation number of bromine is -1. This is because bromine is bonded to one hydrogen atom, which has an oxidation number of +1. The sum of the oxidation numbers of all the atoms in a molecule must be zero, so the oxidation number of bromine must be -1 to balance the positive oxidation number of the hydrogen atom.
The change in oxidation number of bromine from +5 to -1 indicates that bromine has been reduced. This is because bromine has gained electrons in the reaction.
To know more about oxidation number, refer here:
https://brainly.com/question/29100691#
#SPJ11
Nacl has a delta.hfus = 30.2 kj/mol and a delta.hvap = 171 kj/mol. what mass of nacl releases 452 kj of heat as it changes into a liquid?use q equals n delta h..2.64 g22.1 g155 g875 g
Answers
The mass of NaCl released is 154.28 g.
The equation needed to answer this item is already written as q = n(delta)H in the question.
But it was not specified which of the provided heat constants should be utilised. The transition from liquid to gas is the scenario described in this item. Therefore, the heat of vaporisation (delta)Hvap is involved.
The amount of NaCl is calculated through the equation,
n = q/ (delta Hvap)
Substituting the known values,
n = (452 kJ) / (171 kJ/mol)
n = 2.64 mol
Then, we multiply the obtained value by the molar mass of NaCl, 58.44 g/mol.
m = (2.64 mol)(58.44 g/mol)
m = 154.28 g
154.28 g
to learn more about calorimetry go to -
brainly.com/question/14057615?referrer=searchResults
#SPJ4
What are some abiotic factors that ocean creatures near the shore need to be adapted to?
A.salinity levels, air quality, water density
B.wave action, light levels,water depth
C.air quality, salinity levels, light levels
Answers
Answer:A.salinity levels, air quality, water density
Explanation:nba youngboy
Can metals have covalent radius and van der Waal's radius instead of metallic radius?
Answers
Explanation:
Whether you choose to use van der Waals radii or metallic radii as a measure of the atomic radius, for metals the ionic radius is smaller than either, so the problem doesn't exist to the same extent. It is true that the ionic radius of a metal is less than its atomic radius (however vague you are about defining this).
Whether you choose to use van der Waals radii or metallic radii as a measure of the atomic radius, for metals the ionic radius is smaller than either, so the problem doesn’t exist to the same extent. It is true that the ionic radius of a metal is less than its atomic radius (however vague you are about defining this).
HOPE SO IT HELPS YOU
Emily found a piece of metal. She doesn't know what kind of metal it is. What might she measure in lab to find out what
type of metal it is? Is this a physical or chemical property?
Answers
Answer: Emily can measure the density of the piece of metal to find out what it is. Density is a physical property.
Explanation:
The density of a substance can be found by dividing its mass by its volume. No chemical reaction is involved in measuring the mass nor the volume; therefore, density is a physical property.
What is needed to burn the candle (reactant)?
Answers
Answer:
wax, candlewick, and oxygen
Explanation:
The burning of the candle is both a physical as well as a chemical change. The reactants are the substances or the raw materials that are required for a reaction to the process. In the process of burning a candle, the reactants are the fuel which includes wax and wick, and oxygen which is found in the air. The products found at the end of the reaction are carbon dioxide and water vapor.
A beaker has 25 g of snow. The snow melts. What is the mass of the water?
Answers
Answer:
25 millimeters of water
Explanation:
One millimeter of water = when snow melts by 1 gram
hope it helps
2 The name of CICH -CH-CH2OH is A. 1 Chloro propan-3-01 B. 3 chloro propan-1-01 C. 1-chloro propanol D. 3 chloro propanol
Answers
Answer:
3-chloro propan-1-ol
Explanation:
As long as you're writing IUPAC name of a compound, you must mark that the sequence of carbon atoms in the chain from alpha-carbon, which is the carbon right next to the -OH group in this case
There are two isotopes of chlorine. The lighter one with a mass number of 35 (Cl- 35) and the heavier Cl - 37. The atomic mass of chlorine is 35.45 u. Given the mass of chlorine isotopes and the atomic mass of chlorine, determine which isotope is more. Justify your answer.
i need help asap, pls respond quick
Answers
Answer:
Cl-35 isotope is more abundant.
Explanation:
How to calculate the abundance of isotopes in a mixture from the mass of isotopes and the average atomic mass of the element?
The atomic mass of an element having two or more naturally occurring isotopes is calculated using the following relation : Average atomic mass = % abundance of isotope A x atomic mass of isotope A + % abundance of isotope B x atomic mass of isotope B.Solution :
Say the % abundance of Cl - 35 is x, i.e, 100 units of Cl contains x units of Cl-35.
Therefore, the % abundance of Cl - 37 is (100 - x).
∴ [35 x + 37 (100-x)] = 35.45 x 100
Simplifying the above equation, we get
-2x + 3700 = 3545
Subtracting 3700 from both sides of the equation, we get
-2x = -155
or, 2x = 155
Dividing both sides of the equation by 2, we get
x = 155 ÷ 2 = 77.5
∴ 100 -x = 22.5
Thus, Cl-35 is more abundant (77.5%) than Cl-37 (22.5%).
To know more about isotopic abundance, visit:
https://brainly.com/question/24873591
How many elements are Al2O2
Answers
Answer:
i think 2?
Explanation:
AI2 O2
srry if wrong but
hope this helps
Attributes of the genetic code include all of the following except: A. Each codon consists of 3 nucleotides. B. Each codon specifies more than one amino acid. C. Codons are non-overlapping. D. Most am
Answers
The attributes of the genetic code include all of the following except B. Each codon specifies more than one amino acid.
A. Each codon consists of 3 nucleotides: This is a correct attribute of the genetic code. Codons are made up of three consecutive nucleotides, which form the basic unit of the genetic code.
B. Each codon specifies more than one amino acid: This is incorrect. Each codon typically specifies only one amino acid. However, there are some exceptions called "ambiguous codons" where a single codon can code for more than one amino acid, but they are relatively rare.
C. Codons are non-overlapping: This is a correct attribute of the genetic code. Codons are read sequentially and are not overlapping. Each codon starts at a specific position in the DNA or mRNA sequence.
D. Most amino acids are specified by more than one codon: This is a correct attribute of the genetic code. With a few exceptions, most amino acids are encoded by multiple codons. This redundancy provides some level of error tolerance and allows for variations in the DNA sequence without affecting the encoded protein.
learn more about amino acid
https://brainly.com/question/31872499
#SPJ11
anybody who wants to ta lk can come to me.
Answers
Answer:
Ye, whats up bro?
In a titration, 25mL of 0.20M NaOH neutralize 5mL of HCI, what is the acid molarity?
Answers
The molarity of the HCl sample is 1.0 M.
In a titration, the moles of acid are equal to the moles of base at the equivalence point.
We can use this principle to calculate the molarity of the acid (HCl) from the volume and concentration of the base (NaOH) used in the titration.
First, we need to calculate the number of moles of NaOH used in the titration:
moles of NaOH = M x V = 0.20 M x 0.025 L = 0.005 mol
Since NaOH and HCl react in a 1:1 molar ratio, the number of moles of HCl present in the sample is also 0.005 mol.
Now we can calculate the molarity of HCl using the number of moles and the volume of the HCl sample used in the titration:
molarity of HCl = moles of HCl / volume of HCl sample
molarity of HCl = 0.005 mol / 0.005 L
molarity of HCl = 1.0 M
For more question on molarity click on
https://brainly.com/question/14469428
#SPJ11
How many grams of nh3 are needed to provide the same number of molecules as in 0. 65 g of sf6 ?.
Answers
Grams of nh3 are needed to provide the same number of molecules as in 0. 65 g of sf6 is 0.09 grams.
A mole is a very important unit of measurement that chemists use. A mole of something way you have got 602,214,076,000,000,000,000,000 of that thing, like how having a dozen eggs method you've got twelve eggs. Chemists have to degree the use of moles for very small such things as atoms, molecules, or different debris.
calculation:-
Avogadro's number A = 6.022 x 1023
Molecules of NH3 = Molecules of SF6
An x Weight of NH3 / Molecular Weight of NH3
= A x Weight of SF6 / Molecular Weight of SF6
Weight of NH3 = (Weight of SF6 / Molecular Weight of SF6) x Molecular Weight of NH3
= (0.75 / 146.05) x 17.03 = 0.09 grams
The weight of NH3 needed is 0.09 grams
The mole, image mol, is the unit of amount of substance within the international system of devices. the quantity of the substance is a measure of what number of elementary entities of a given substance in an item or sample. The mole is defined as containing exactly 6.02214076×10²³ basic entities
Learn more about moles here:-https://brainly.com/question/15356425
#SPJ4
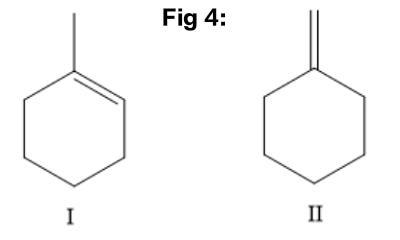
Draw a structural formula of an alkene or alkenes (if more than one) that undergo acid-catalyzed hydration and without rearrangement give 1- methylcyclobutanol as the MAJOR product. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • If more than one structure fits the description, draw them all. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu.
Answers
The structure of 1-methyl cyclohexanol is: shown in Fig 1
The reactions for the preparation of 1-methyl cyclohexanol are summarised as: Fig 2
Here In Fig 2, 1-methyl cyclohexene reacts to give 1-methyl cyclohexanol in the presence of water. It proceeds through acid catalysed hydration.
In the Fig 3 reaction, methylene cyclohexane reacts with water to give 1-methyl cyclohexanol. It proceeds through acid catalysed hydration.
Thus, the possible structures of alkene are depicted as: Fig 4
Learn more about Alkene here ; https://brainly.com/question/13910028?referrer=searchResults
#SPJ4



if you use too much hot solvent when dissolving your crude compound, how will that impact the recovery of your compound and why?
Answers
Recrystalization will occur.The solution may become too diluted for crystals to form if you add too much solvent. Impurities will be captured by a hastily formed crystal's lattice. The crystals that result will also be smaller.
What is Recrystalization?Recrystallization is a physical process used to separate compounds based on how soluble they are. Heating the material to dissolve the compound with impurities in a mixture of a suitable solvent completes the procedure. We can remove the desired chemical or contaminants from the mixture using this method.
The solution may become too diluted for crystals to form if you add too much solvent. The flask needs to be gently cooled, first at room temperature and then in cold water. Impurities will be captured by a hastily formed crystal's lattice. The resulting crystals will also be smaller.
This method is used to harden steel in order to eliminate all strain hardening side effects, including the significant plastic deformation brought on by cold working.The crystals that frequently form when the compound precipitates out gave it its name. The natural expansion of larger ice crystals at the expense of smaller ones is another definition of recrystallization.Some commonly effective mixes include diethyl ether-methanol (or ethanol) for polar molecules (particularly esters, alcohols, and hydrocarbons) and diethyl ether-petroleum ether (or benzene) for strongly linked solids (notably amides, alcohols), as well as many natural products.The three main types of recrystallization are;
Single-solvent recrystallization.Multi-solvent recrystallization.Hot filtration-recrystallization.To know more about Recrystalization, refer to:
https://brainly.com/question/10194206
#SPJ1
PLEASE HELP WHAT IS THE RIGHT ANSWER

Answers
Answer:
C
Explanation:
Weak acids dissociate only slightly in an aqueous solution. The majority of molecules remain undissociated.
what type of intermolecular forces does ammonium lauryl sulfate have?
Answers
Answer:
Ammonium lauryl sulfate, like any other surfactant, makes a good base for cleansers because of the way it disrupts the hydrogen bonding in water. Hydrogen bonding is the primary contributor to the high surface tension of water. In hydrogen bonding with the water surrounding them.
What is the name of this compound CH3CH(CH3)CH3
Answers
The Correct option is A, The IUPAC name of the compound CH3–CHCH3–CO–CH3 is 3-methyl-2-butanone.
In chemistry, a compound is a substance formed by the chemical combination of two or more different elements in fixed proportions. The atoms in a compound are held together by chemical bonds, which can be covalent, ionic, or metallic depending on the nature of the elements involved.
Compounds have unique physical and chemical properties that are different from their constituent elements. For example, water is a compound formed by the chemical combination of hydrogen and oxygen in a fixed ratio of 2:1 by mass. While hydrogen is a highly flammable gas and oxygen is necessary for combustion, water is a non-flammable liquid that is essential for life.
There are many different types of compounds, including organic and inorganic compounds. Organic compounds are those that contain carbon atoms, while inorganic compounds do not. Examples of organic compounds include sugars, proteins, and fats, while examples of inorganic compounds include salt, water, and carbon dioxide.
To learn more about Compound visit here:
brainly.com/question/19458442
#SPJ4
Complete Question:
The IUPAC name of the compound CH3–CHCH3–CO–CH3 is
A 3-methyl-2-butanone
B 2-methyl-3-butanone
C Isopropyl methyl ketone
D 2ethyl-2methyl pentane
A major industrial use of hydrochloric acid is in metal pickling. This process involves the removal of metal oxide layers from metal surfaces to prepare them for coating.
Write a balanced equation between iron (III) oxide and hydrochloric acid to form iron (III) chloride and water.
How many moles of water would you expect to produce if 7.25 moles of hydrochloric acid reacted completely?
How many atoms of hydrogen are present in the moles of water calculated in part b)?
If 2.11 moles of iron (III) oxide and 275.3 g of hydrochloric acid react, how many grams (maximum) of iron (III) chloride will be produced? (Hint: find the limiting reactant)
How many grams of the excess reactant is left over?
Answers
The major industrial use of hydrochloric acid is in metal pickling, which involves the removal of metal oxide layers from metal surfaces to prepare them for coating.
The balanced equation between iron (III) oxide and hydrochloric acid to form iron (III) chloride and water is:Fe2O3 + 6HCl → 2FeCl3 + 3H2OIf 7.25 moles of hydrochloric acid reacted completely, we would expect to produce 10.875 moles of water. There are 21.75 atoms of hydrogen present in this amount of water.To determine the maximum amount of iron (III) chloride that can be produced, we need to find the limiting reactant. Using the given amounts, the number of moles of hydrochloric acid is calculated to be 6.819 moles, while the number of moles of iron (III) oxide is 2.11 moles. Therefore, iron (III) oxide is the limiting reactant. The maximum amount of iron (III) chloride that can be produced is 639.76 g.To calculate the amount of excess reactant left over, we need to find the amount of hydrochloric acid that was not used up in the reaction. Using the balanced equation, we can calculate that 1 mole of Fe2O3 reacts with 6 moles of HCl. Therefore, 2.11 moles of Fe2O3 would require 12.66 moles of HCl. However, we only had 6.819 moles of HCl, so there is 5.84 moles of HCl left over. Converting to grams using the molar mass of HCl, we get that 275.3 g of HCl - 149.53 g of HCl = 125.77 g of excess HCl.
For more similar questions on topic Stoichiometry.
https://brainly.com/question/14935523
#SPJ11
What are the ionic compounds of gunpowder within fireworks, and what would be the chemical reaction of its combustion?
Answers
There are a lot of ionic compounds in fireworks, most of them are salts, like: Strontium Nitrate and Strontium Carbonate ( both in the color red); Calcium Carbonate and Calcium Chloride (orange); Sodium Nitrate, Sodium Oxalate (yellow); Barium Nitrate and Barium Carbonate (green) and so on. For the gunpowder itself we have the Potassium Nitrate as an ionic compound.
The chemical reaction for the combustion of black powder is:
6KNO3 + C7H4O + 2S -> K2CO3 + K2SO4 + K2S + 4CO2 + 2CO + 2 H2O + 3N2
It's not just a single reaction, but this is a general representation of it.
draw the structures and give the names of the simplest straight-chain (the triple bond between c1 – c2) alkynes containing seven to twelve carbons.
Answers
The simplest straight-chain alkynes containing seven to twelve carbons are:
1. Heptyne (7 carbons)
2. Octyne (8 carbons)
3. Nonyne (9 carbons)
4. Decyne (10 carbons)
5. Undecyne (11 carbons)
6. Dodecyne (12 carbons)
Here are the structures and names of the simplest straight-chain alkynes containing seven to twelve carbons:
1. Heptyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Heptyne
2. Octyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Octyne
3. Nonyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Nonyne
4. Decyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Decyne
5. Undecyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Undecyne
6. Dodecyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Dodecyne
Learn more about alkynes at https://brainly.com/question/27937735
#SPJ11
A twin-turbojet airplane is cruising with a speed of Mach 1.5 at an altitude where atmospheric pressure is 32989.5 Pa, temperature is 232.778 K. Each engine is consuming 200 kg of air per second. The engine exit flow has a pressure of 32,000 Pa with a velocity of 850 m/sec. The exit area of the engine nozzle is 1.4 m
2
. How much thrust both engines are generating?
Answers
A twin-turbojet airplane is cruising with a speed of Mach 1.5 at an altitude where atmospheric pressure is 32989.5 Pa, temperature is 232.778 K. Each engine is consuming 200 kg of air per second. The engine exit flow has a pressure of 32,000 Pa with a velocity of 850 m/sec. The exit area of the engine nozzle is 1.4 m². The thrust generating in both engines is 342,678.6 Newtons.
To calculate the thrust generated by both engines, we can use the momentum equation for a nozzle:
Thrust = mass flow rate * exit velocity + (exit pressure - ambient pressure) * exit area
Given:
Speed of the airplane (V) = Mach 1.5
Atmospheric pressure (\(P_a\)) = 32989.5 Pa
Ambient temperature (\(T_a\)) = 232.778 K
Mass flow rate of each engine (m) = 200 kg/s
Exit pressure of the engine (\(P_e\)) = 32000 Pa
Exit velocity of the engine (\(V_e\)) = 850 m/s
Exit area of the engine nozzle (\(A_e\)) = 1.4 m²
First, we need to calculate the ambient density using the ideal gas law:
PV = nRT
Since the speed of the airplane is given in terms of Mach number, we can calculate the speed of sound (a) using the following formula:
a = √(gamma * R * \(T_a\))
Where gamma is the specific heat ratio of air (approximately 1.4) and R is the specific gas constant for air (approximately 287 J/(kg K)).
Next, we can calculate the ambient density (ρ) using the equation:
ρ = \(P_a / (R * T_a)\)
Now, we can calculate the thrust generated by each engine using the momentum equation:
Thrust = m* \(V_e + (P_e - P_a) * A_e\)
Finally, we can calculate the total thrust generated by both engines by multiplying the thrust of a single engine by 2.
Calculate the speed of sound:
a = √(1.4 * 287 * 232.778)
a = 438.95 m/s
Calculate the ambient density:
ρ = 32989.5 / (287 * 232.778)
ρ = 1.383 kg/m³
Calculate the thrust of a single engine:
\(Thrust_s\) = 200 * 850 + (32000 - 32989.5) * 1.4
\(Thrust_s\) = 170000 + 1339.3
\(Thrust_s\) 171339.3 N
Calculate the total thrust of both engines:
\(Thrust_t\) = 2 * \(Thrust_s\)
\(Thrust_t\) = 2 * 171339.3
\(Thrust_t\) = 342678.6 N
Therefore, both engines are generating approximately 342,678.6 Newtons of thrust.
To know more about thrust here
https://brainly.com/question/26712174
#SPJ4
What do these two changes have in common?
crushing a mineral into powder
picking up a paper clip with a magnet
Select all that apply.
Both are changes of state.
Both conserve mass.
Submit
Both are only physical changes.
Both are chemical changes.

Answers
The appearance and observable qualities of matter are considered to be its physical attributes. Colour, smell, taste, solubility, etc. An attribute that appears during a chemical reaction is known as a chemical property. A few examples include pH, reactivity, and flammability, etc. The correct option is B.
The chemical makeup or content of matter are not altered after a physical transformation. The internal makeup is unaffected as molecules rearrange themselves during this transformation. The chemical attribute is unaffected by a physical change.
Here both crushing a mineral into powder and picking up a paper clip with a magnet are physical changes.
Thus the correct option is B.
To know more about physical changes, visit;
https://brainly.com/question/28742279
#SPJ1
part 1: name the type of chemical reaction that occurs when calcium hydroxide (ca(oh)2) reacts with
nitric acid (hno3).
part 2: explain why zinc (zn) would react with lead nitrate (pb(no3)2) but not with calcium chloride
(cacl2).
Answers
The type of reaction that occurs between calcium hydroxide and nitric acid is a neutralization reaction.
What is a neutralization reaction?The term neutralization reaction refers to a reaction that occurs between an acid and a base that leas to the formation of salt and water only. The type of reaction that occurs between calcium hydroxide and nitric acid is a neutralization reaction.
The reason why zinc will react with lead nitrate and not calcium chloride is because lead is less than zinc in the electrochemical series hence it can be displaced from solution.
Learn more about neutralization reaction:https://brainly.com/question/20038776
#SPJ1
Please show how you solved :)
What is oxygen solubility at 10m depth below sea level, 25 deg
C, 30 g/L salinity?
Answers
The solubility of oxygen at 10m depth below sea level, 25 degrees Celsius, and 30 g/L salinity is approximately 6.59 mg/L.
To calculate the solubility of oxygen at a specific depth below sea level, temperature, and salinity, we can refer to the oxygen solubility tables. The solubility of oxygen can vary depending on these factors.
1. Begin by identifying the given parameters:
- Depth: 10m below sea level
- Temperature: 25 degrees Celsius
- Salinity: 30 g/L
2. Use the given parameters to locate the corresponding values in the oxygen solubility table.
3. The solubility of oxygen at a depth of 10m below sea level, 25 degrees Celsius, and 30 g/L salinity is typically around 6.59 mg/L.
Therefore, the solubility of oxygen at 10m depth below sea level, 25 degrees Celsius, and 30 g/L salinity is approximately 6.59 mg/L.
Learn more about solubility from this link:
https://brainly.com/question/9098308
#SPJ11
The oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity is approximately 1538 mol/L.
To calculate the oxygen solubility at a specific depth below sea level, temperature, and salinity, we can use the solubility formula.
The solubility of a gas decreases with increasing temperature and salinity, and increases with increasing pressure.
Here's how you can calculate the oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity:
1. Determine the pressure at 10m depth below sea level: -
The pressure at sea level is approximately 1 atmosphere (atm).
The pressure increases by approximately 1 atm for every 10 meters of depth.
Therefore, at 10m depth, the pressure is approximately 2 atm.
2. Convert the temperature to Kelvin: -
To convert from Celsius to Kelvin, add 273 to the temperature.
25°C + 273 = 298 K.
3. Use the solubility formula:
The solubility of oxygen in water can be calculated using Henry's law:
S = k * P * C.
S is the solubility of oxygen in moles per liter (mol/L).
k is the Henry's law constant for oxygen in water at a specific temperature and salinity.
P is the partial pressure of oxygen in atmospheres (atm).
C is the concentration of oxygen in moles per liter (mol/L).
4. Look up the Henry's law constant for oxygen at 25°C and 30 g/L salinity:
The Henry's law constant for oxygen at 25°C and 30 g/L salinity is approximately 769 L*atm/mol.
5. Calculate the solubility:
S = (769 L*atm/mol) * (2 atm) * (1 mol/L). - S ≈ 1538 mol/L.
Therefore, the oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity is approximately 1538 mol/L.
Learn more about solubility from this link:
brainly.com/question/9098308
#SPJ11
what is the percent yield when a reaction vessel that initially contains 61.5 kg ch4 and excess steam yields 13.0 kg h2
Answers
The percent yield of the reaction is 84.62%.
How to calculate the percent yield of a reaction?To calculate the percent yield of a reaction, you need to know the theoretical yield and the actual yield of the product.
In this case, the balanced equation for the reaction between CH4 and steam (H2O) is:
CH4 + 2H2O → CO2 + 4H2
From the equation, we can see that for every mole of CH4 reacted, we should get 4 moles of H2 produced.
To determine the theoretical yield of H2, we need to convert the given mass of CH4 to moles and then use the mole ratio from the balanced equation to calculate the expected amount of H2 produced.
Molar mass of CH4 = 16 g/mol
Number of moles of CH4 = 61,500 g / 16 g/mol = 3843.75 mol
From the balanced equation, 1 mole of CH4 produces 4 moles of H2.
So, the expected moles of H2 = 3843.75 mol x 4 = 15375 mol
The actual yield of H2 is given as 13.0 kg = 13,000 g.
Now, we can calculate the percent yield using the following formula:
Percent yield = (Actual yield / Theoretical yield) x 100%
Plugging in the values we obtained above, we get:
Percent yield = (13,000 g / 15375 mol) x 100%
= 84.62%
Therefore, the percent yield of the reaction is 84.62%.
Learn more about percent yield
brainly.com/question/2506978
#SPJ11
a 10.000-l tank of nitrogen contains 4.000 moles of gas at 22.00c. calculate the expected pressure if neon was an ideal gas
Answers
The pressure of the gas can be determined using ideal gas equation. The pressure of neon in the tank is 9.7 atm.
What is ideal gas equation ?Ideal gas equations states the relation between volume, pressure, temperature and number of moles of gas as written below:
PV = nRT.
Where R is the universal gas constant equal to 0.082 L atm/K mol.
Given the volume of the tank V = 10 L
number of moles n = 4 moles
temperature = 22 ° C = 295 k
Then pressure P = nRT/V.
Pressure of neon gas = 4 moles × 295 K× 0.082 L atm/K mol /(10 L)
P = 9.7 atm.
Therefore, the pressure of the neon gas in the tank is 9.7 atm.
Find more on ideal gases:
https://brainly.com/question/30247106
#SPJ1
2. In pigeons, the allele B produces ash-red feathers. The allele b produces
blue feathers. The B allele is dominant to the ballele. A pigeon with
genotype Bb is crossed with a pigeon with genotype bb. What percent of
the offspring are expected to have ash-red feathers?
A. 0%
B. 25%
C. 50%
D. 100%
Answers
Answer:
it is C
Explanation:i had this on my test and got it right lol