HURRRYYY
in a chemical reaction you will always have an excess reactant. true or false?
Answers
Answer:
It's False
Explanation:
In a chemical reaction, reactants that are not used up when the reaction is finished are called excess reagents. The reagent that is completely used up or reacted is called the limiting reagent, because its quantity limits the amount of products formed.
Hope this helps you
Answer: false
Explanation:
Related Questions
how to find boiling point given delta h and delta s
Answers
The boiling point of a substance can be found by using the equation: T = (delta h / delta s), where delta H is the enthalpy change and delta S is the entropy change.
To find the boiling point of a substance given the enthalpy change (delta h) and entropy change (delta s), we can use the equation:
delta G = delta H - T * delta S
Here, delta G represents the change in Gibbs free energy, T is the temperature in Kelvin, delta H is the enthalpy change, and delta S is the entropy change.
The boiling point is the temperature at which the Gibbs free energy change becomes zero, indicating that the substance is transitioning from a liquid to a gas. To find the boiling point, we rearrange the equation:
delta G = delta H - T * delta S
Solving for T:
T = (delta H / delta S)
By substituting the given values of delta H and delta S into the equation, we can calculate the boiling point of the substance.
Learn more:About find here:
https://brainly.com/question/22188924
#SPJ11
A closed system is one which no matter can enter or exit. True or false
Answers
False. In a closed system, matter can not enter or exit that is there is no change in the matter of the system.
Three types of systems exist in nature:
1. Open System: In this system, the matter can interact with the surroundings or matter can enter or exit the system from the surrounding. Similarly, the energy of the system also interacts with its surroundings and can be lost or gained.
For example oceans etc.
2. Closed system: In this system, the matter is unable to interact with the surroundings that are matter can't exit or enter the system. While the energy of the system is able to interact with the surroundings.
For example Earth etc
3. Isolated system: In this system, both matter and energy are unable to interact with the surrounding. There is no exchange between matter and the energy of surroundings.
For example thermos-teel bottles etc.
Learn more about Open Systems:
https://brainly.com/question/28891854
#SPJ4
4. After 15 minutes, another solution is added to the reaction flask and the % Transmittance returns to 100%. Did another reaction take place? Please explain your answer.
Answers
Based on the graph and the information provided, it can be stated another solution took place as there is a change in the properties of the substance.
What is transmittance?The word "transmittance" refers to the ratio or in this case the percentage of light that can pass through a substance of object. This concept implies that if the tranmittance is 100% is beceuse 100% of the light is passing through the sample and 0% is of the light is being absorbed by it. In the same way if the transmittance is 20% is because the solution has likely become more dense and 80% of the light is being absorbed.
What happened if the transmittance returns to 100%?Considering the transmittance is directly related to concentration and density if there is a big change in the transmittance such as this returning to 100% it is likely because there was a chemical reaction that changed the properties (density) or concentrion of the sample.
Learn more about substances in: https://brainly.com/question/24372098
#SPJ1

The compound M-O-H can act both as acid or base depending upon the ionisation
enthalpy of the element M. Justify by taking elements (M) of the third period of the
periodic table.
action
Answers
Answer:
Explanation:
A compound that can act both as an acid and a base is called an amphoteric substance. A good example (based on the question) of an amphoteric substance is the Aluminium hydroxide [Al(OH)₃] where Aluminium is the "M" in M-O-H. Aluminium is in the third period of the periodic table. The reaction below shows aluminium can act both as an acid and as a base in different neutralization reactions.
As an acid
Al(OH)₃ + 3NaOH ⇒ Na₃AlO₃ + 3H₂O
As a base
3HCl + Al(OH)₃ ⇒ AlCl₃ + 3H₂O
NOTE: A neutralization reaction is a reaction between an acid and a base to give you salt and water
Which of the following items describe a mole? a. Avogadro's number of items b. 6.022 multiply 10^23 items c. mass multiply acceleration d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon.
Answers
The correct descriptions of a mole are:
a. Avogadro's number of items
b. 6.022 × \(10^2^3\) items
d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon.
a. Avogadro's number of items:
Avogadro's number is a fundamental constant in chemistry and is defined as the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. It is approximately equal to 6.022 × \(10^2^3\) items. Therefore, option a correctly describes a mole as Avogadro's number of items.
b. 6.022 × \(10^2^3\) items:
This is the numerical value of Avogadro's number. As mentioned earlier, it represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. So, option b is another correct description of a mole.
c. Mass multiplied by acceleration:
This description does not accurately describe a mole. The product of mass and acceleration is a measure of force (Newton's second law of motion) and is unrelated to the concept of a mole in chemistry.
d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon:
This is a correct description of a mole. It refers to the concept of the molar mass, where one mole of a substance contains the same number of particles (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This concept allows for the conversion between mass (in grams) and the number of moles.
So, the correct options are a, b, and d.
To know more about Avogadro's number, refer
here:https://brainly.com/question/28812626
#SPJ4
Which one of the following salts, when 1 mole is dissolved in water, produces the solution with a pH closest to 7.00? NaOH LiF KCl NH4Cl
Answers
The salt that, when 1 mole is dissolved in water, produces the solution with a pH closest to 7.00 is NH4Cl.
This is because NH4Cl is an acidic salt that undergoes hydrolysis in water to produce ammonium ions (NH4+) and chloride ions (Cl-). The ammonium ions can act as a weak acid, reacting with water to form hydronium ions (H3O+), which decreases the pH of the solution.
However, the chloride ions can act as a weak base, reacting with water to form hydroxide ions (OH-), which increases the pH of the solution. The net effect is that the pH of the solution is slightly acidic, but closest to 7.00.
Among the given salts, when 1 mole is dissolved in water, potassium chloride (KCl) produces a solution with a pH closest to 7.00. This is because KCl dissociates into potassium (K+) and chloride (Cl-) ions in water, and neither ion significantly affects the pH of the solution.
To know more about hydrolysis: brainly.com/question/11461355
#SPJ11
What is a key difference between chemical and nuclear reactions?
A. In chemical reactions, new compounds are formed. In nuclear reactions, compounds are destroyed.
B. Chemical reactions involve electron rearrangements. Nuclear reactions involve changes to the nucleus.
C. Chemical reactions involve large changes in energy. Nuclear reactions absorb or release small amounts of energy.
D. In chemical reactions, only alpha radiation is emitted. In nuclear reactions, alpha, beta, and gamma decay may occur.
Answers
Answer:
B
Explanation:
Chemical reactions involve electron rearrangements. Nuclear reactions involve changes to the nucleus.
Answer:
B
Explanation:
A slightly edited Exercise 6 of Chapter 4 (Page 90) states:
(a) Calculate the energy needed to bring a cup of water (about 250 g) from 10°C to the boiling point (100°C for water). Then, find the time it takes to heat this water (c) in a 1-kg aluminum pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. Assume the pan, too, starts at 10°C and has to be heated to water’s boiling point.
Solution:
(a) To heat just the water requires energy Qw=mwcwΔT (Equation 4.3), where ΔT=100∘C−10∘C=90∘C:
Qw=0.25kg(4184Jkg∘C)90∘C=94,140J
(c) On the stove, we also have to heat the pan. Aluminum’s specific heat is ca=900Jkg∘C , from table 4.3, (because this is lower than cw, it is easier to heat aluminum than water).
To heat just the aluminum pan requires energy, Qa=macaΔT=1kg(900Jkg∘C)90∘C.
The total energy to heat the pan of water on the stove is increased because of the finite efficiency:
Qtotal=Qw+Qaes=94,140J+81,000J0.75=233,520J
The time it takes to heat the water depends on the stove’s power: power = energy per time, so
t=energypower=QtotalPs=233,520J1,500Js=155.68or156sonthestove
Question:
Find the time, in seconds, it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. Assume the pan, too, starts at 10°C and has to be heated to water’s boiling point. Round your answer to the nearest whole second.
Answers
The time it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan is 90 seconds (rounded to the nearest whole second).
We need to calculate the time taken to heat the water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. The given information are as follows:
Specific heat of water, cw = 4184 J/kg °C
Specific heat of steel, cs = 450 J/kg °C
Energy supplied by the electric stove burner, P = 1,500 W (75% of which is transferred to the water and the pan)
Mass of water, mw = 250 g = 0.25 kg
Mass of steel pan, ms = 1 kg
Initial temperature of water and steel pan, T1 = 10 °C
Final temperature of water and steel pan (boiling point of water), T2 = 100 °C
Heat absorbed by the steel pan = Qs = ms × cs × (T2 - T1)Heat absorbed by the water = Qw = mw × cw × (T2 - T1)
Total heat absorbed by the water and the pan = Q = Qw + Qs = (0.25 × 4184 × 90) + (1 × 450 × 90) J= 94,140 + 40,500 J= 1,34,640 J
Time taken to heat the water and the pan = t = Q/P= 1,34,640 / 1,500 s= 89.76 or 90 s
Therefore, the time it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan is 90 seconds (rounded to the nearest whole second).
To know more about Mass, visit:
https://brainly.com/question/11954533
#SPJ11
Write a message to Eric Wu explaining when he can photograph a lunar eclipse and why lunar eclipses happen.
Claim 1: A lunar eclipse can be photographed any time Earth is in between the sun and the Moon.
Claim 2: A lunar eclipse can be photographed sometimes when Earth is in between the sun and the Moon.
Answers
A lunar eclipse can be photographed sometimes when Earth is in between the sun and the Moon.
Why lunar eclipse occur?A lunar eclipse occurs when the Sun, Earth, and Moon align in the same line in which the Moon passes into Earth's shadow. In a total lunar eclipse, the entire Moon falls within the darkest part of Earth's shadow, called the umbra so we can conclude that claim 2 is the right answer about lunar eclipse.
Learn more about eclipse here: https://brainly.com/question/1077760
Hypothesis for candy bar density lab
Need answer ASAP!!!!!
Answers
dissolved load is moved by . a. saltation b. suspension c. traction d. solution
Answers
Dissolved load is moved by traction. Dissolved load refers to particles or substances that are suspended in a fluid and are carried along by the fluid's movement. Option C.
Traction is the force that is exerted on an object by a fluid, such as water or air, and it can be used to move dissolved loads. Other mechanisms that can be used to move dissolved loads include saltation (the process of bouncing along the surface of a fluid), suspension (the process of being carried along in a fluid), and solution (the process of being dissolved in a fluid). However, traction is the most common mechanism for moving dissolved loads.
Learn more about Dissolved
https://brainly.com/question/2364287
#SPJ4
Full Question;
dissolved load is moved by .
a. saltation
b. suspension
c. traction
d. solution
Ordinary hydrogen contains 99.30% of H atoms and 0.70% H atoms. Calculate the relative atomic mass of hydrogen.
Answers
The relative atomic mass of hydrogen 1.00
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit
Here given data is
99.30% of H atoms
0.70% H atoms
So we have to calculate the relative atomic mass of hydrogen. = ?
So the relative atomic mass = (isotope abundance × isotope mass) + (isotope abundance × isotope mass)
Relative atomic mass = (99.30% × 1) + (0.70% × 1)
Relative atomic mass = 1.00
Know more about hydrogen
https://brainly.com/question/25640729
#SPJ9
A high viscosity liquid will
Answers
Answer: high viscosity fluids move sluggishly and resist deformation.
Explanation: some examples of high viscosity liquids is Honey, syrup, motor oil, and other liquids that do not flow freely
What is the main function of this organ system It protects vital organs from injury. It is the first line of defense and keeps the body warm. It transports nutrients and oxygen to body cells. It produces hormones that regulates growth and metabolism.
Answers
Answer:
Organ systems & their main functions
Explanation:
The chest bones Rib Cage & spine - protects the vital organs (heart, lung,liver) from injury & provides structural support for body.
Skin is the body's first line of defense & keeps the body warm. In case of any invading infection, fever - body temperature rise fights it.
Circulatory system (containing heart) & its body fluid blood - transports nutrients and oxygen to body cells
Endocrine system regulates growth, produces hormones - that target organs via bloodstream.
predict how the reaction rate would change if the concentration of the sodium meta-bisulfite solution were changed instead of the potassium iodate
Answers
The reaction rate would increase.
In an acidic media, the reaction between potassium iodate and sodium meta-bisulfite produces iodine. Firstly, hydrogen sulfite and iodate ions are formed. Iodide ions are produced as a result of the reaction between iodate ions and hydrogen sulfite ions. Iodine is formed when the excess of iodate ions reacts with iodide ions in the existence of hydrogen ions. Iodine instantly combines with any remaining hydrogen sulfite ions to make iodide ions before it can mix with starch to form a dark blue-colored complex. The iodine is then free to combine with the starch to create the colorful complex after all of the hydrogen sulfite ions have completed their reactions. The color reveals the consumption of the sulfite ions. In order to observe this reaction, a known but constrained amount of sodium meta-bisulfite solution and starch solution can be added. The amount of time it takes for the blue color to develop is used to measure the rate of reaction. Overuse of bisulfite will stop the development of the dark blue complex.
You can learn more about titration by visiting the following link;
https://brainly.com/question/2728613
#SPJ4
pls answer this asap thankyou
Balance the chemical equation using linear algebra technique. PC15 + H₂O → H3PO4 + HCl 1) Create a matrix or put in vector form by the assignment of the variables P CI given: 1 PCl5 + 2 H₂O3 H3PO4 + 24 HCl i.e. â = H O 2) Set the equation zero and show your augmented matrix form. Ac = Ô 3) Solve the system using row operations and simplify the solutions (assign a convenient value if a free variable exists to express solutions as set of integers). 4) Enter the coefficients into the chemical equation: PC15 + H₂O H3PO4 + HCI
Answers
To balance the chemical equation PC15 + H2O → H3PO4 + HCl using linear algebra technique, we can set up an augmented matrix and solve the system of equations. Here's the step-by-step process:
Create a matrix or put it in vector form by assigning variables to P, Cl, H, O: Let's assign:
P = x (coefficient for PCl5)
Cl = y (coefficient for HCl)
H = z (coefficient for H3PO4)
O = w (coefficient for H2O)
The equation becomes: xPCl5 + yH2O → zH3PO4 + wHCl Set up the equation in matrix form:
| PCl5 | + | H2O | = | H3PO4 | + | HCl |
| 1 | + | 0 | = | 0 | + | 0 |
| 0 | + | 2 | = | 0 | + | 0 |
| 0 | + | 0 | = | 1 | + | -1 |
| 0 | + | 0 | = | 0 | + | 24 |
This gives us the augmented matrix form:
| 1 0 0 0 0 |
| 0 1 2 0 0 |
| 0 0 0 1 -1 |
| 0 0 0 0 24 |
Solve the system using row operations: Performing row operations to simplify the augmented matrix:
R2 = R2 - 2R1
R4 = R4 / 24
The simplified augmented matrix becomes:
| 1 0 0 0 0 |
| 0 1 0 0 0 |
| 0 0 0 1 -1 |
| 0 0 0 0 1 |
From the simplified matrix, we can assign values to the variables:
P = 0
Cl = 0
H = 0
O = 0
This implies that the coefficients for PCl5, H2O, H3PO4, and HCl are all zero. Enter the coefficients into the chemical equation: The balanced chemical equation is:
0PCl5 + 0H2O → 0H3PO4 + 0HCl
Therefore, the balanced equation is: PCl5 + H2O → H3PO4 + HCl (unbalanced)
To learn more about chemical, https://brainly.com/question/28792948
#SPJ11
A student is designing a new insulated drink cup using unconventional materials. They will have an inside and an outside cup with a material from the table in between the cups as insulation.Which material should they use to prevent heat loss?
Answers
The best material for insulation in this case would be Styrofoam. Styrofoam is lightweight, strong, and an excellent thermal insulator. It is composed of tiny bubbles of air that are suspended in a matrix of plastic. The air trapped inside the bubbles acts as a thermal barrier, keeping heat out or in, depending on the application.
Its lightweight nature makes it easier to manipulate, while its strength gives it the durability needed to keep a drink hot or cold. Its insulation properties also make it the perfect material for the student's insulated drink cup.
Styrofoam can be cut and shaped easily, making it a great material for use in drink cups. The material is also easy to clean and resistant to water and other liquids, which makes it ideal for frequent use. Additionally, Styrofoam is both affordable and widely available, making it an ideal choice for the student's project.
Know more about thermal insulator here:
https://brainly.com/question/23134662
#SPJ11
The density of lead is 11.3g/ml. What is the volume in ml of 50.75g of lead?
Answers
Answer:
4.49 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\\)
From the question we have
\(volume = \frac{50.75}{11.3} \\ = 4.491150...\)
We have the final answer as
4.49 mLHope this helps you
One likely reason some experimental automobiles have been developed to use electricity rather than gasoline is that
Answers
One likely reason some experimental automobiles have been developed to use electricity rather than gasoline is to reduce carbon emissions and increase energy efficiency.
Gasoline-powered vehicles emit greenhouse gases that contribute to climate change, while electric vehicles produce zero emissions. Additionally, electric vehicles have a higher energy efficiency than gasoline-powered vehicles because they convert a higher percentage of the energy stored in their batteries into motion, whereas gasoline engines waste a significant amount of energy as heat. These factors make electric vehicles a more environmentally friendly and efficient alternative to traditional gasoline-powered vehicles.
Electric vehicles produce zero tailpipe emissions, which significantly reduces air pollution and greenhouse gas emissions. In addition, they are more energy-efficient because they convert a higher percentage of energy from their power source into vehicle movement, while gasoline-powered cars lose a significant amount of energy as waste heat. Furthermore, the electricity used to charge EVs can be generated from renewable sources like solar or wind, which further reduces their environmental impact.
To know more about energy visit:
https://brainly.com/question/8630757
#SPJ11
Be sure to answer all parts. Compounds a and b are isomers having molecular formula c5h12. Heating a with cl2 gives a single product of monohalogenation, whereas heating b under the same conditions forms three constitutional isomers. What are the structures of a and b?.
Answers
Neo-pentane represents the Compound A while compound B is n-pentane.
After careful consideration we can say that compounds A and B are alkanes and also isomers of pentane. In chemistry, Isomers are defined as compounds having same empirical molecular formula but different structural formulas due to varying arrangement of atoms.
Now, as per the question statement, compound A gives a single monochlorination product upon heating with the molecule of chlorine i.e. Cl2 showing that the molecule is extremely symmetric. This molecule must be neo-pentane. Refer to image 1.
Similarly, Compound B forms 3 constitutional isomers after undergoing monochlorination. This compound must be n-pentane since three are 3 different types of carbon atoms in the structure. Refer to image 2.
If you need to learn more about neo-pentane click here:
https://brainly.com/question/20815247
#SPJ4


Neopentane makes up component A, while n-pentane makes up compound B.
First and foremost, it is important to understand that compounds A and B are isomers and alkanes of pentane. Compounds with distinct structural formulas but the same molecular formula are known as isomers.
When heated with Cl2, compound A now produces a single monochlorination product, demonstrating the molecule's high degree of symmetry. Neopentane must be this chemical (image 1).
Upon monochlorination, compound B divides into three constitutional isomers.
A halogen atom is replaced with another substance in a process known as halogenation, where the halogen atom eventually becomes a component of the new substance or compound. In general, one or more halogens are typically added to the chemical during the halogenation reaction.
Learn more about halogens:
https://brainly.com/question/14191541
#SPJ4
One molecule of silver atoms contains the same number of items found in one mole of calcium atoms. TrueFalse
Answers
When they talk about items found, they refer to molecules. According to Avogadro, one mole of any substance, regardless of its type, contains the same amount of molecules equal to 6.022x10^23.
Now they tell us about a molecule of silver and a mole of calcium. In one mole of calcium, there will be 6.023x10^23 molecules, which is different from one molecule of silver, so the answer will be false
How Many Of The Following Molecules Are Polar? XeCl_2 COF_2 PCl_4F SF_6 A) 2 B) 0 C) 1 D) 4
Answers
D) 4. 4 Molecules Are Polar in XeCl_2 COF_2 PCl_4F SF_6. While the molecule's S-F bonds are polar, it is nonpolar due to its octahedral shape and lack of lone pairs.
The existence of polar bonds and the molecular geometry affect a molecule's polarity. When the electronegativity of the atoms making the bond differs significantly, the bond is said to be polar. To ascertain each molecule's polarity:
XeCl2: Because of the difference in electronegativity, Xe-Cl bonds are polar, but because the molecule is linear, it is not polar.
COF2: It is polar because the C-O and C-F bonds are polar and the molecular geometry is trigonal planar.
PCl4F: The molecule has a tetrahedral shape with one lone pair, which makes the P-Cl bonds in the compound polar.
SF6: While the molecule's S-F bonds are polar, it is nonpolar due to its octahedral shape and lack of lone pairs.
The (C)1 molecule (COF2) is polar, thus that is the correct response.
learn more about molecule here:
https://brainly.com/question/19922822
#SPJ4
35. a
When aqueous iron (III) chiondes added to aqueous potassium iodide a chemical con
ours and lodine is formed
Which statement is correct?
A todide sons are oxidised, they gain electrons in this reaction
lodide ions are oxidised, they lone electrons in this reaction
C trond) chionde is oxidised in this reaction
D Neither iodide ions nor iron (III) chlonde is ondised in this reachon

Answers
What is the neutral subatomic particle that is a part of the nucleus?
Answers
Answer
A neutron
Explanation:
Give any two reasons why we need to separate things in our daily life.
Answers
Answer:
1: They could get mixed up and then it messes other stuff up, 2: It keeps you orgnized
Explanation:
The Thermite reaction reacts iron (III) oxide, Fe2O3 with aluminium powder, Al, to form aluminium oxide, Al2O3 and iron, Fe. Fe2O3 + 2Al ➔ Al2O3 + 2Fe a. A student reacted 16.0g of iron (III) oxide with 8.1g of aluminium powder.
a.Which of the two reactants is the limiting reagent? Show your working.
b. Calculate the maximum mass of iron that could be formed using these quantities of reactants.
Answers
I sincerely hope this isn't wrong

The mass of the iron will produce is equal to 11.16 grams and iron oxide will be the limiting reagent.
What is a limiting reagent?A limiting reagent can be explained as the reactant present in the chemical reaction which is consumed completely first during the completion of a reaction.
The limiting reagent in a chemical reaction decides the amount of the product when the reactants are not taken in stoichiometry.
Given, chemical reaction of iron oxide and aluminum represented as:\(Fe_2O_3 + 2Al \longrightarrow Al_2O_3 + 2Fe\)is:
The mass of the iron oxide = 16 g
The number of moles of iron oxide = 16/159.7 = 0.1 moles
The mass of aluminum powder = 8.1 g
The number of moles of Al = 8.1/27 = 0.3 mol
The 2 moles of Al react with iron oxide = 1
0.3 moles of Al react with iron oxide = 0.3/2 = 0.15 mol
Therefore iron oxide is a limiting reagent.
One mole of iron oxide will produce iron = 2 mol
0.1 mol of iron oxide will produce iron metal = 2 × 0.1 = 0.2 mol
The maximum mass of iron metal = 0.2 ×55.8 = 11.16 g
Learn more about limiting reagent, here:
brainly.com/question/11848702
#SPJ2
A sample of a gas has a volume of 0.102 dm3 at a temperature of 201 K. If the temperature is doubled, what will be the new volume
Answers
Plz help, have a quiz on this plz PLZ

Answers
Answer:
20.82
Explanation:
Assuming that the trends continue, which of the following compound will have the greatest solubility at 120 ℃? The graph below shows the solubility of a variety of compounds.
A. Ce2(SO4)3
B. K2Cr2O7
C. NaCl
D. Pb(NO3)2
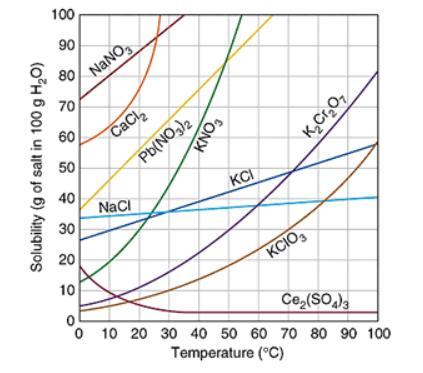
Answers
Answer:
its c
Explanation:
i took the test and got a 100
Assuming that the trends continue, sodium chloride compound will have the greatest solubility at 120 ℃.
What is solubility?Solubility is defined as the ability of a substance which is basically solute to form a solution with another substance. There is an extent to which a substance is soluble in a particular solvent. This is generally measured as the concentration of a solute present in a saturated solution.
The solubility mainly depends on the composition of solute and solvent ,its pH and presence of other dissolved substance. It is also dependent on temperature and pressure which is maintained.Concept of solubility is not valid for chemical reactions which are irreversible. The dependency of solubility on various factors is due to interactions between the particles, molecule or ions.
Learn more about solubility,here:
https://brainly.com/question/22185953
#SPJ3
A prokaryotic cell does not have
А
a cell membrane.
B
genetic material.
C
a nucleus.
D
flagella.
Answers
Answer:
D
Explanation: