Answers
The balanced redox equation is 2FeS₂(s) + 11Na₂O₂(s) + 5H₂O(l) → 2Fe2O₃(s) + 11Na₂SO₄(s) + 4NaOH(aq).
How to balance to a redox reaction?To balance a redox equation, you need to follow these steps:
Write out the unbalanced equation.Assign oxidation states to each element in the equation.Identify the species that are oxidized and reduced, and determine the change in oxidation state for each.Balance the atoms in the equation, excluding oxygen and hydrogen.Balance the oxygen atoms by adding water molecules to the appropriate side of the equation.Balance the hydrogen atoms by adding hydrogen ions (H+) to the appropriate side of the equation.Balance the charge by adding electrons (e-) to the appropriate side of the equation.Check that the number of atoms and the charge are balanced on both sides of the equation.Here is the balanced redox equation for the reaction between FeS₂ and Na₂O₂:
2FeS₂(s) + 11Na₂O₂(s) + 5H₂O(l) → 2Fe2O₃(s) + 11Na₂SO₄(s) + 4NaOH(aq)
In this equation, FeS₂ is oxidized and Na₂O₂ is reduced. The Fe in FeS₂ goes from an oxidation state of +2 to +3, and the O in Na₂O₂ goes from -1 to -2.
The equation is balanced by adjusting the coefficients of each species. The final equation has 2FeS₂, 11Na₂O₂, and 5H₂O on the left side and 2Fe₂O₃, 11Na₂SO₄, and 4NaOH on the right side. The equation is balanced in terms of both atoms and charge.
Learn more on redox reaction here: https://brainly.com/question/26263317
#SPJ1
Related Questions
find the ph of a mixture of 10.0 ml of 0.00100 m solution of potassium hydroxide, koh and10.0 ml of distilled water.
Answers
The pH of a mixture of 10.0 ml of 0.00100 M solution of potassium hydroxide, KOH, and 10.0 ml of distilled water is 11.00.
To calculate the pH of the mixture using the formula for pH which is:
pH = -log[H⁺]
First, to find the concentration of hydroxide ions [OH⁻] in the given solution using the formula for the concentration which is given as follows:
Concentration (molarity) = Moles of solute/ volume of solution in Litres
Number of moles of KOH = molarity x volume
= 0.00100 mol/L x 0.0100 L = 1.00 x 10⁻⁵ mol
Concentration of KOH = 1.00 x 10⁻⁵5 mol / 0.010 L = 0.00100 M
For a neutral solution, the concentration of H⁺ ions is equal to the concentration of OH- ions.
Concentration of OH⁻ ions = 0.00100 M (since KOH is a strong base, it dissociates completely in water)
For a strong base like KOH,
pOH = -log[OH-] = -log (0.00100) = 3.00
Now, let's find pH using the formula for pH which is given below:
pH + pOH = 14.00
pH = 14.00 - pOH
pH = 14.00 - 3.00
pH = 11.00
Therefore, the pH of a mixture is 11.00.
Learn more about pH: https://brainly.com/question/2288405
#SPJ11
PLEASE HELP 100 POINTS!!!! AND GIVING BRAINLIEST!!!!!!!!
Why should a movie producer consider filming a new version of The First Men in the Moon? Create a 6-8 slide multimedia presentation to market your idea for this movie to a production company. Rubric Criteria Points Possible 20 (10) 11-19 (6-9) 6-10 (3-5) 0-5 (0-2) Points Earned Ideas & Content Relates Theme of Novel Provides analysis of character Accurate diagram of plot 20 Establishes a theme, relates an analysis of the protagonist, effectively summarizes the plot, and maintains a clear focus throughout. Establishes a theme, identifies some character traits of the protagonist, provides a summary of the plot, and maintains focus throughout. There are a few lapses in focus, but the theme is fairly clear. Presents somewhat of an analysis of character. The plot summary is mostly correct. It is difficult to figure out the theme of the novel. The character analysis is poor or not present. The plot summary does not accurately represent the story. Organization Structure Precision Focus 20 Strong organization; seamless transitions between ideas; Effective and precise content. Organization is appropriate but conventional; Attempt at a focused and precise sales pitch. Attempts at organization, inappropriate use of lists or bullets; content is unfocused and ideas are not developed No clear organizational framework or transitions. Ideas are vague or unfocused Voice Personality Sense of audience 20 Strong awareness of audience in the design. Students can clearly explain why they felt the vocabulary, audio and graphics chosen fit the target audience. Some awareness of audience in the design. Students can partially explain why they felt the vocabulary, audio and graphics chosen fit the target audience. Some awareness of audience in the design. Students find it difficult to explain how the vocabulary, audio and graphics chosen fit the target audience. Limited awareness of the needs and interests of the target audience. Images and Music Relevance to theme Effectiveness Imagery 10 Images and music stir a rich emotional res
Answers
Answer:-Science have researched more facts and cool things about it-It would have more facts and things to learn-The quality would be better and more understandingI just tried 3 quick ideas I hope it can help a bit
Explanation:
Answer:
the answer is you should know this already :)))))))))))))))))))))))))))))))))))))))))))))))))))
The unequal sharing of electrons within a water molecule is called: ________
Answers
The unequal sharing of electrons within a water molecule is called a polar covalent molecule.
Polar covalent molecule is defined as the molecule which have unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two poles which is a positive charge on the hydrogen pole (side) and a negative charge on the oxygen pole (side).
A polar covalent bond is defined as a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. Sometimes in a polar covalent bond, which is simply called as a polar bond, the distribution of electrons around the molecule remains no longer symmetrical.
Learn more about polar covalent molecule from the link given below.
https://brainly.com/question/1906476
#SPJ4
Which of the following distinguishes hydrogen bonds from covalent bonds? a.) Only covalent bonds can form between molecules. b.) Only hydrogen bonds can form within molecules. c.) Only hydrogen bonds can form between molecules. d.) Only ionic bonds can form within molecules.
Answers
Only hydrogen bonds can form between molecules distinguishes hydrogen bonds from covalent bonds. Hence option (d) is the answer.
How do you distinguish between hydrogen bonds and covalent bonds?When atoms distribute their electrons evenly to form a molecule, a covalent link is created. An atom and the positive charge of a hydrogen atom that is covalently bonded to another substance are attracted to one another by electrostatic forces, creating a hydrogen bond. It is weaker than a covalent link and an intermolecular bond.
Atoms of two or more come together and share electrons to form covalent bonds. By either gaining or losing an electron, these atoms combine in an effort to find stability. When a hydrogen atom that is partially positive is drawn to an atom that is partially negatively charged, hydrogen bonds are created. Ionic bonds occur when one atom transfers one or more electrons to another atom.
To learn more about covalent bonds, visit:
https://brainly.com/question/4461443
#SPJ1
Describe the properties of carbon that makes it possible for millions of carbon compounds to occur naturally.
Please help! will mark brainliest!!
Answers
Some of the properties of carbon that make it possible for millions of carbon compounds to occur naturally include:
catenationformation of multiple bondsease to react with other elements such as hydrogen, oxygen, and nitrogenWhat are carbon compounds?Carbon compounds are compounds that are composed mainly of carbon bonded to other elements.
Carbon compounds may be organic or inorganic in nature.
Carbon due to some of its unique properties is responsible for the existence of many compounds in living organisms known as organic compounds.
Some of the unique properties of carbon include:
catenation - carbon is able to link to other carbon atoms to form long chains of organic compounds.ability to form either single, double, or triple bonds.Learn more about organic compounds at: https://brainly.com/question/6279332
#SPJ1
ir fingerprint region analysis i. what peaks are present that should be present? ii. what peaks are absent that should be absent? iii. are there any peaks present or absent that should not be?
Answers
The fingerprint region is important because different compound produces different pattern of troughs on the spectrum.
a)A broad peak in the region between 3100 and 3600 cm-1 shows the presence of exchangeable protons from alcohol, amine, amide or carboxylic acid groups.
b)When sample does not absorb any radiation then all the radiation is transmitted and sample has 100% transmittance. Hence, absorptions in the IR spectrum are as downward deflection that is an “upside-down peak.
b)Homonuclear diatomic molecules do not show IR spectra because they do not have a permanent dipole moment nor the stretching of the atoms about the bond gives any dipole moment
Triggering of molecular vibrations through irradiation with infrared light is called Infrared Spectroscopy. It provides information about the presence or absence of certain functional groups.
The range of IR absorption for covalent bonds is 600 - 4000 cm-1.
An IR -spectrum shows peaks from the range of 3600 to 500 cm-1. IR -frequencies corresponding to the molecular vibrations frequencies.
IR spectra is called fingerprint region because absorption pattern is quite complex but unique for each organic structure. The vibrations for both the carbon-carbon and carbon-oxygen double bonds are identified at 6.1 and 5.8 μm, respectively.
To know more about Infrared Spectroscopy, refer
https://brainly.com/question/28543039
#SPJ4
If a yellow paint is to have 0.511% pbcro4 by mass, how many grams of chromite are needed per kilogram of paint?
Answers
Yellow paint has 0.511 % PbCrO4 by mass
Mass of PbCrO4 in 1 kg of paint = (0.511 / 100) * 1 kg = 0.00511 kg = 5.11 g
Moles of PbCrO4 = 5.11 g/ 323.19 g/mol
= 0.0158 moles
Moles of K2CrO4 also = 0.0158 moles
Moles of FeCr2O4 = 0.0158 moles K2CrO4 * (4 mole FeCr2O4 / 8 moles K2CrO4)
= 0.0079 moles
Mass of FeCr2O4 (chromite) = 0.0079 moles * 223.83 g/mol
= 1.77 g
Paints are divided into two types: oil-based paints and water-based paints. Oil paints are usually used as a primer, undercoat, and topcoat. In the past, we relied heavily on oil-based paints for surface durability and longevity, but today water-based paints are on par. It is used for prolongation and acts as a barrier against environmental influences. Paint consists of pigments, solvents, resins, and various additives. Pigments give the paint its color. Solvents make application easier. The resin helps dry. Additives range from fillers to antifungals. There are hundreds of different natural and synthetic pigments.
Learn more about paint here
https://brainly.com/question/17996239
#SPJ4
True or
False?
Consider the equilibrium c(s) h2o(g) co(g) h2(g), δh = 2296 j. the concentration of carbon monoxide will increase if the temperature of this system is raised.
Answers
In the given reaction, the concentration of carbon monoxide will increase if the temperature of this system is raised. The given statement is true.
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
H₂O + CO ⇄ H₂ + CO₂
The equilibrium will shift to the right direction i.e towards products.
If the temperature of the system is increased, the concentration of carbon dioxide is increased , so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of takes place. Therefore, the equilibrium will shift in the right direction i.e. towards the products.
To know more about equilibrium here
https://brainly.com/question/28166356
#SPJ4
_Cl2 + _NaBr → _NaCl + _Br2
Answer: 1,2,2,1
Answers
Can somebody plz help and these t or f questions correctly (only if u really know the correct ones) thx sm! :3
WILL MARK BRAINLIEST WHOEVER ANSWERS FIRST :DDDD

Answers
2.false
3.true
4. True
5.false
I hope that helps :)
Ice at 0.0°C is mixed with 7.30 × 10^2 mL of water at 25.0°C. How much ice must melt to lower the water temperature to 0.0°C? The specific heat capacity of water is 4.186 J/(g·K). Latent heat of fusion for water is 333.7 J/g.
Answers
Approximately 35.90 grams of ice must melt to lower the water temperature to 0.0°C.
To solve this problem, we need to calculate the amount of heat that needs to be transferred from the water to the ice in order to lower the water temperature to 0.0°C.
First, let's calculate the initial heat content of the water. The specific heat capacity of water is 4.186 J/(g·K), and the mass of the water can be calculated using its density (1 g/mL) and volume (7.30 × 10^2 mL):
Mass of water = density × volume = 1 g/mL × 7.30 × 10^2 mL = 7.30 × 10^2 g
The initial heat content of the water can be calculated using the formula:
Heat content = mass × specific heat capacity × temperature change
Heat content = 7.30 × 10^2 g × 4.186 J/(g·K) × (25.0°C - 0.0°C) = 7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C
Next, we need to calculate the amount of heat that needs to be transferred from the water to the ice to lower the water temperature to 0.0°C. This heat transfer occurs during the melting of the ice.
The amount of heat required to melt the ice can be calculated using the formula:
Heat = mass of ice melted × latent heat of fusion
Let's assume that x grams of ice melts. The mass of the ice can be calculated using its density (0.92 g/mL) and volume (same as the volume of water):
Mass of ice = density × volume = 0.92 g/mL × 7.30 × 10^2 mL = 6.716 × 10^2 g
Heat = x g × 333.7 J/g
Now, we need to ensure that the heat transferred from the water to the ice is enough to lower the water temperature to 0.0°C. The heat transferred from the water to the ice is equal to the heat transferred from the water when its temperature drops to 0.0°C:
Heat content of water = Heat transferred to ice
7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C = x g × 333.7 J/g
Now, we can solve for x:
x = (7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C) / (333.7 J/g)
x ≈ 35.90 g
Therefore, approximately 35.90 grams of ice must melt to lower the water temperature to 0.0°C.
Learn more about temperature
https://brainly.com/question/27944554
#SPJ11
What type of imf are between nonpolar covalent molecules? polar covalent molecules? ionic compounds? water?.
Answers
The type of IMF between nonpolar covalent molecules is the London dispersion force. The type of IMF between polar covalent molecules is dipole-dipole force. The type of IMF between water molecules is hydrogen bonding.
The London dispersion force is a result of electron movement and results in the formation of temporary dipoles in molecules. This happens because the movement of the electrons in nonpolar molecules isn't uniform; it's constantly fluctuating. As a result, there will be areas where electrons are more concentrated than others, resulting in temporary positive and negative charges.
Polar covalent molecules have dipole-dipole force. It's due to the partial positive charge on one molecule interacting with the partial negative charge on another molecule.
Ionic compounds have ionic bonds, and it's an electrostatic attraction between positively and negatively charged ions.
Water molecules have hydrogen bonding. The molecules in the water are held together by hydrogen bonds, which are strong IMF.
You can learn more about covalent molecules at: brainly.com/question/14262966
#SPJ11
How many valence electrons will the cation Al2+ have?
Answers
Answer:
Aluminum has an atomic number of 13. The electronic configuration of Al is 1s2 2s2 2p6 3s2 3p1. Al3+ has only 10 electrons so the configuration of Al 3+ is 1s2 2s2 2p6. The highest energy level is 2 so the number of electrons in the highest level is the valence electrons which is 8.
Answer:
Answer is Below
Explanation:
According to , Since the last shell of an aluminum-ion has eight electrons, the valence electrons of aluminum ion (Al3+) are eight.
Hopes this Helps :D
A molecule is the smallest part of
A an element
B a compound
& a substance
D an atom
Answers
Answer: a substance
Explanation:
A molecule is the smallest part of a substance
What is Al and O's balanced compound?
Answers
Answer:
Aluminum generally forms Al3+ ions. Oxygen generally forms O2− ions. So when the metal and the non-metal make music together they form a neutral compound, i.e. Al2O3 (i.e. 2×3−3×2=0) . The ionic bonding is so strong (due to charge magnitude) in this material that it is reasonably insoluble.
Explanation:
Can anybody help me with this?

Answers
This model of an atom was developed by Ernest Rutherford, a New Zealand native working at the University of Manchester in England in the early 1900s. Rutherford spent most of his academic career researching aspects of radioactivity and, in 1908, won the Nobel Prize for his discoveries related to radioactivity. It was after this that Rutherford began developing his model of the atom.
Based on their electrons dot diagrams, what is the formula for the covalently bonded compound
of nitrogen and hydrogen?
Answers
Answer:
Nitrogen, the next nonmetal, has 5 electrons in the valence shell, so it needs to combine with 3 hydrogen atoms to fulfill the octet rule and form a stable compound called ammonia (NH3).

The covalent compound formed from nitrogen and hydrogen is called ammonia with the formula NH₃. Each hydrogen shares its valence electron with three valence electrons of nitrogen.
What is ammonia?Ammonia, NH₃ is a covalent compound formed by the combination of nitrogen and three hydrogen atoms. Nitrogen is a highly electronegative element with 5 valence electrons.
Nitrogen needs 3 more electrons to achieve octet. Hence valency of nitrogen is 3. During chemical bonding it gains three electrons though sharing or donation from metals.
Hydrogen needs one more electron to be stable. Hence, nitrogen shares its three valence electrons each with three hydrogens and each hydrogen in turn shares its one valence electron with nitrogen forming NH₃.
Find more on ammonia:
https://brainly.com/question/15409518
#SPJ2
HELP Sometimes a retired military vessel is hauled out to sea and
intentionally sunk. After some time on the seafloor, the ship begins to
form a coral reef that is home to many sea creatures. Which of the
following best describes how this action affects the environment?
A
The number of predators in the area is decreased.
B
A new habitat is created that supports several kinds of life.
с
Many harmful toxins are introduced into the environment.
D
Plants and animals have a new source of energy to live.
Answers
Answer:
probably B, a new habitat is created that supports several kinds of life.
Explanation:
I wasn't here to learn this can someone help me

Answers
A debate among scientists on how new species form is based on speciation.
Divergence explains how organisms on Earth are related because it shows how species are similar or different.
Genes play the role in diversity of carrying information that results in changes from one generation to the next.
Natural selection is part of evolution because it explains why attributes are either in existence or have gone extinct.
What do scientists debate about how species are formed ?One of the main debates among scientists on how new species form centers around the concept of speciation. There are two main hypotheses for speciation: allopatric speciation and sympatric speciation.
Divergence is the process by which different populations or species of organisms evolve from a common ancestor over time. This process is responsible for the diversity of life on Earth. By studying the similarities and differences between different organisms, scientists can infer evolutionary relationships.
Genes are the basic units of inheritance that carry information from one generation to the next. They play a crucial role in creating diversity among organisms. The genetic diversity of a population is the result of mutations, gene flow, and genetic drift.
The theory of natural selection is one of the most important and widely accepted explanations for the diversity of life on Earth. It is the process by which certain traits become more or less common in a population over time, depending on their impact on the survival and reproduction of the organisms that possess them.
Find out more on natural selection at https://brainly.com/question/23929271
#SPJ1
help pls i will give 15 point

Answers
a man with mass 60kg climbs up staircase having 30 step each of 10cm height in 10seconds.Calculate his power.
Answers
Answer:
Power = Work done * time
P = (F * d) * t
P = (mg * d) * t
d = 1 step = 10 cm
30 steps = 300 cm
300 cm = 3m
P = (60*9.8 * 3) * 10
P = 17640 Watt
P = 1.764 Kilowatt
Ans: 60 watt
Explanation:
Solution
M=60kg, G=10
Distance=10*10/100
=1m
Time=10sec
Now,
W=M×G×H
=60×10×1
=600
P=w÷t
=600÷10
=60watt
Hence, the power of the man is 60watt
please help!! im taking the test now
Which lists the elements in order from least conductive to most conductive?
A. nitrogen (N), antimony (Sb), bismuth (Bi)
B. nitrogen (N), bismuth (Bi), antimony (Sb)
C. antimony (Sb), nitrogen (N), bismuth (Bi)
D. bismuth (Bi), antimony (Sb), nitrogen (N)
Answers
Answer:
b
Explanation:
Answer: nitrogen (N), antimony (Sb), bismuth (BI)
Explanation:
The elements listed belongs to Group V on the periodic table.
during chemiosmosis in aerobic respiration, protons are pumped __________.
Answers
Electrons are passed through a series of redox reactions, and each transfer causes protons to be pumped across the membrane. This creates a concentration gradient, which is used to power ATP synthesis through the process of chemiosmosis.
During chemiosmosis in aerobic respiration, protons are pumped across the inner mitochondrial membrane from the matrix to the intermembrane space.
Aerobic respiration is a process of producing energy that involves the complete breakdown of glucose in the presence of oxygen. It is a crucial metabolic pathway that is present in all higher organisms, including humans.Chemiosmosis is the process in which a transmembrane electrochemical gradient drives ATP synthesis. It is an important part of cellular respiration and oxidative phosphorylation.
During the process of oxidative phosphorylation, protons are pumped across the inner mitochondrial membrane, which creates a proton gradient that powers the synthesis of ATP. In aerobic respiration, the electron transport chain (ETC) is the primary mechanism that generates the proton gradient.
Electrons are passed through a series of redox reactions, and each transfer causes protons to be pumped across the membrane. This creates a concentration gradient, which is used to power ATP synthesis through the process of chemiosmosis.
to know more about Aerobic respiration visit :
https://brainly.com/question/11874459
#SP11
Mercury poisoning is a debilitating disease that is often fatal. In the human body, mercury reacts with essential enzymes leading to irreversible inactivity of these enzymes. If the amount of mercury in a polluted lake is 0.4 Hg/mL, what is the total mass in kilograms of mercury in the lake
Answers
Answer:
The total mass of mercury in the lake is 631,542.7 kg
Explanation:
Question: The given dimensions of the lake as obtained from a similar question posted online are;
The surface area of the lake, A = 100 mi²
The lake's average depth, d = 20 ft.
The concentration of the mercury, C = 0.4 μg Hg/mL = 0.4 × 10⁻⁶ kg Hg/L
Therefore, we have;
The volume of water mixture in the lake, V = A × d
∴ V = 100 mi² × 20 ft. = 2,787,840,000 ft.² × 20 ft. = 55,756,800,000 ft.³
1 ft³ = 28.31685 L
∴ 55,756,800,000 ft.³ = 55,756,800,000 ft.³ × 28.31685 L/ft.³ = 1.57885675 × 10¹² L
The total mass of mercury in the lake, m = C × V
∴ m = 0.4 × 10⁻⁶ kg Hg/L × 1.57885675 × 10¹² L = 631,542.7 kg
The total mass of mercury in the lake, m = 631,542.7 kg.
What is the mass of 72cm of silver if the density is 10.5g/cm
Answers
Answer:
756g
Explanation:
density x volume = mass
The density of the substance can be used to estimate the mass or the volume of the substance. The mass of 72 cm³ volume of silver with a density of 10.5 g/cm³ will be 756 g.
What is the relationship between mass and density?The mass of the substance is said to be directly proportional to the density of the substance. It is given as D ∝ m.
The mass of the substance can be given by multiplying the density of the object with the volume. The formula for mass is given as,
Mass = Density × volume
Given,
Density (D) = 10.5 g/cm³
Volume of silver (V) = 72 cm³
Substituting values of density and volume above,
Mass = Density × Volume
= 10.5 g/cm³ × 72 cm³
= 756 gm
Therefore, 756 grams is the mass of 72 cm³ volume of silver with a density of 10.5 g/cm³.
Learn more about mass and density here:
https://brainly.com/question/1624312
#SPJ2
Which phrase best describes body composition?
a way to manage body weight
the percentage of fat in the body
the entire makeup of the human body
substances in equal amounts in the body
Answers
Answer:
4th phrase
substances in equal amounts in the body
Answer:
D
Explanation: (:
An object that has potential energy may have this energy because of its
a acceleration
b location
c momentum
d speed
Answers
Answer:
i have no idea. let's say location because it has to be somewhere.
Explanation:
I
how many atoms are in 4(NH3)2NO2?
Answers
There are a total of 38 atoms in the compound 4(NH3)2NO2.
How to find the number of atomsTo determine the number of atoms in the given compound, we first need to break down the formula and identify the number of atoms in each element.
The formula is 4(NH3)2NO2, which can be written as:
4 x 2(NH3) + 1 x 2(NO2)
Each molecule of NH3 contains
one atom of nitrogen and three atoms of hydrogenEach molecule of NO2 contains
one atom of nitrogen and two atoms of oxygen.Therefore, the total number of atoms in the given compound is:
4 x 2 x (1 nitrogen atom + 3 hydrogen atoms) + 1 x 2 x (1 nitrogen atom + 2 oxygen atoms)
= 4 x 2 x 4 + 1 x 2 x 3
= 32 + 6
= 38
Learn more about atoms at
https://brainly.com/question/6258301
#SPJ1
If 80mg of a radioactive element decays to 10 mg in 30 mins., what is the element's half-life in minutes?
a- 10 b- 20 c- 30 d- 40
Answers
Answer:
the answer is a.10
Explanation:
in(No/N) = kt
No = 80 mg
N = 10 mg
t = 30 min
solve for k, then
k = 0.693/t1/2
Substitute k from above and solve for t1/2
10 min is correct.
Radioactive element keeps on decaying over the time. Decay of radioactive element always comes under first order kinetics. The element's half-life of decay of 80mg of a radioactive element decays to 10 mg in 30 mins is 10.04 minutes. The correct option is option A.
What is half life?Half life tells about the time at which the radioactive material decays to half of its initial concentration.
Mathematically, the initial and final amount of radioactive material can be connected as
ln(No/N) = kt
No =initial amount of radioactive decay= 80 mg
N = final amount of radioactive decay=10 mg
t =time taken to decay= 30 min
K= rate constant
ln( 80 mg/10 mg) = k×30 min
0.069min⁻=k
k = 0.693/half life
half life=10.04min
Therefore the element's half-life of decay of 80mg of a radioactive element decays to 10 mg in 30 mins is 10.04 minutes. The correct option is option A.
To know more about half life, here:
brainly.com/question/21464063
#SPJ2
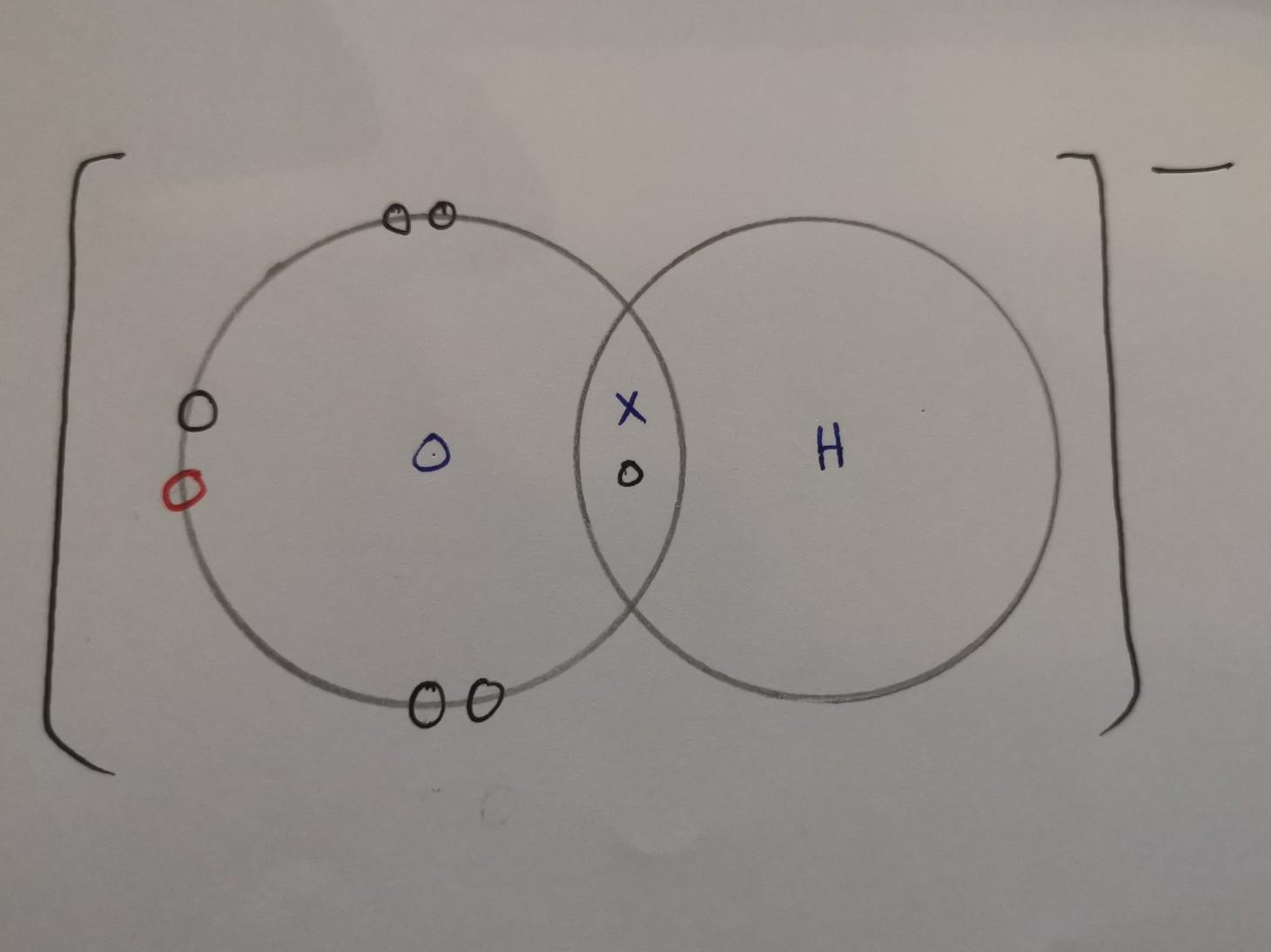
Draw the electron dot structure of the hydroxide ion (OH-).
Answers
Answer:
X = electrons from hydrogen
O (black) = electrons from oxygen
O (red) = electrons from when it was connected to a metal atom, hence why it has a negative charge