Answers
The specific heat capacity of a substance, also known as the massic heat capacity or massic heat capacity in thermodynamics, is the heat capacity of a sample of the substance divided by the mass of the sample. So, the energy gain by sample will be 506J
We know that, the heat energy gained will be calculated by the formula,
Q=mcΔT
where,
Q= Heat energy gained
m=mass
c= specific heat capacity
ΔT= Change in temperature
So, Given,
m= 5.00g ,
ΔT= 419K -306K= 113K
c= 0,897J/gK
Q=mcΔT
Q= 5.0×0.897×113
Q= 506J
So, the energy gained by sample of Aluminum will be 506 J when it is warmed from 306 K to 319K , in which temperature difference is 113K
To know more about specific heat capacity, please refer:
https://brainly.com/question/27991746
#SPJ9
Related Questions
Write the equilibrium constant expression for this reaction: 2H²(aq)+SO₁¯(aq) → H₂SO4(aq)
Answers
The reaction's equilibrium constant is 2.4 x 1047 + 500 K for the reaction 2H2(g) + O2(g) 2H2O(g).
What is the 2NO2 g N2O4 g equilibrium constant expression?Assuming a standard free energy change of 5.40 kJ/mol, the equilibrium constant for the reaction N2O4(g)2NO2(g) N 2 O 4 (g) 2 N O 2 (g) is 0.113 at 298 K.
At a certain temperature, Kc = 2.4 x 103 molL m o l L is the equilibrium constant for the reaction 2NON2+O2 2 N O N 2 + O 2.
The ideal gas law is expressed as PV=nRT, where P is pressure, V is volume, n is number of moles in the substance, R is constant at 0.08206 L atm/K mol, and T is temperature in Kelvin.
learn more about equilibrium constant
https://brainly.com/question/3159758
#SPJ1
What limitations occurs for chalk in vinegar chemistry pd lab experiment?
Also the precautions to take
Need this asap!!
Answers
Answer:
When conducting a chemistry lab experiment using chalk (calcium carbonate) in vinegar (acetic acid), there are several limitations and precautions to be aware of:
Limitations of chalk in vinegar chemistry experiment:
Reaction rate: The reaction between chalk and vinegar is relatively slow, which may require a longer observation period or higher concentration of vinegar to observe significant changes within a reasonable time frame.
Solubility: Chalk may not dissolve completely in vinegar, resulting in incomplete reaction or difficulty in obtaining accurate results.
Product formation: The reaction between chalk and vinegar produces carbon dioxide gas, water, and calcium acetate. The carbon dioxide gas may escape into the atmosphere, leading to loss of product and inaccurate measurements.
pH: Chalk is a basic substance, and the reaction with vinegar, which is acidic, may result in neutralization, leading to a decrease in the overall acidity of the reaction mixture.
Precautions to take in chalk in vinegar chemistry experiment:
Ventilation: The reaction between chalk and vinegar produces carbon dioxide gas, which can displace air and potentially cause asphyxiation in a closed or poorly ventilated area. Conduct the experiment in a well-ventilated area or under a fume hood to ensure adequate air circulation.
Eye and skin protection: Vinegar is an acid and can cause skin and eye irritation. Wear appropriate personal protective equipment (PPE), such as gloves and goggles, to protect yourself from contact with vinegar or any other chemicals used in the experiment.
Chemical handling: Handle the chemicals, including chalk and vinegar, with care, following proper lab safety protocols. Avoid ingestion, inhalation, or direct contact with the chemicals, and dispose of them properly according to local regulations.
Accuracy in measurements: Use calibrated and accurate measuring tools, such as graduated cylinders or burettes, to measure the amount of chalk, vinegar, and other reagents accurately. This will ensure the reliability and accuracy of the experimental results.
Observations: Make careful and detailed observations during the experiment, noting any changes in appearance, gas evolution, or other relevant observations. Take measurements at appropriate intervals and record the data accurately for analysis and interpretation.
It is important to follow good laboratory practices, including proper chemical handling, accurate measurements, and cautious observations, to ensure safe and reliable results in a chalk in vinegar chemistry lab experiment. Consult with a qualified instructor or supervisor for specific guidelines and precautions related to your experiment.
Which two statements about composite materials is true?
A. They're made up of more than one substance
B. They have the same or similar properties as the materials used to make them
C. They're always made of metal
D. They're readily available in nature
help as fast as u can please
Answers
What is the name of the following elements: Na, K, Br, O, Fe?
Answers
Answer:
Na = Sodium
K = Potassium
Br = Bromine
O = Oxygen
Fe = Iron
Explanation:
The approximate diameter of the spherical nucleus of hydrogen-1 atom is 1 x 10^-13 cm. Estimate the density (g/cm3) of matter in a proton of mass of proton is 1.673 x 10^-24
Answers
The diameter of the spherical nucleus of hydrogen-1 atom is 1 x 10^-13 cm. The density (g/cm3) of matter in a proton of mass of proton is 1.673 x 10^-24 is 3.193 × 10¹⁵ g/cm³.
What is density ?
The density of a substance is its mass per unit volume. The most common symbol for density is, but the Latin letter D can also be used. Density is defined mathematically as mass divided by volume.
Diameter = 1 x 10⁻¹³ cm
Mass = 1.673 x10⁻²⁴ g
Volume = 4/3πr³
Volume = 4/3 × 22/7 × (1 × 10⁻¹³ / 2)³
Volume = 11 × 10⁻³⁹ / 21
Density = Mass / Volume
= 1.673 x10⁻²⁴ / 11 × 10⁻³⁹ / 21
Density = 3.193 × 10¹⁵ g/cm³
Thus, the density (g/cm3) of matter in a proton of mass of proton is 1.673 x 10^-24 is 3.193 × 10¹⁵ g/cm³.
To learn more about the density, follow the link;
https://brainly.com/question/29775886
#SPJ9
What are the causes and consequences of climate change
Answers
Answer: Humans are increasingly influencing the climate and the earth's temperature by burning fossil fuels, cutting down forests and farming livestock.
Explanation: This adds enormous amounts of greenhouse gases to those naturally occurring in the atmosphere, increasing the greenhouse effect and global warming.
The decomposition of HI(g) at 298 K is represented by the equilibrium equation above. When 100. torr of HI(g) is added to a previously evacuated, rigid container and allowed to reach equilibrium, the partial pressure of I2(g) is approximately 3.7 torr. If the initial pressure of HI(g) is increased to 200. torr and the process is repeated at the same temperature, which of the following correctly predicts the equilibrium partial pressure of I2(g), and why?
answer choices
PI2 ≈ 14 torr, because it is directly proportional to the square of the initial pressure of HI.
PI2 ≈ 0.073 torr, because it is inversely proportional to the square of the initial pressure of HI.
PI2 ≈ 7.4 torr, because it is directly proportional to the initial pressure of HI.
PI2 ≈ 1.9 torr, because it is inversely proportional to the initial pressure of HI.
Answers
7.4 torr for PI2. Due to its direct proportionality to the initial pressure of HIHI, PI2 7.4 torrs.
The forward and reverse reactions happen simultaneously when the system is in equilibrium. Each reactant and product are present in equal amounts once equilibrium has been attained. The relationship between the concentrations of the reactants and the products is mathematically represented by the equilibrium constant equation.
The equilibrium state is the state of a system whose attributes are constant under constant external conditions. Even after equilibrium has been reached, reactants and products continue to change, making chemical equilibria dynamic in nature. However, the reaction time for moving forward and backward is the same. Balance examples include Placed on the table was a book. a vehicle that travels at a fixed speed. a chemical process where the rates of the forward and reverse reactions are equivalent.
Learn more about equilibrium here:
brainly.com/question/517289
#SPJ4
What is/are the purpose(s) of adding HCl to the reaction mixture?
Answers
The addition of hydrochloric acid to a reaction mixture can serve several purposes, depending on the specific reaction and its conditions such as: (1) To provide protons (H⁺), (2) To adjust the pH of the reaction mixture, (3) To remove impurities or byproducts.
Why hydrochloric acid is added to a reaction mixture?(1) To provide protons (H⁺) for acid-catalyzed reactions: HCl is a strong acid, meaning it readily donates protons to other molecules. In some reactions, the presence of HCl can accelerate the reaction by increasing the concentration of protons in the reaction mixture. This can facilitate bond-breaking and bond-forming steps in the reaction mechanism.
(2) To adjust the pH of the reaction mixture: In some reactions, it may be necessary to maintain a specific pH range for the reaction to proceed optimally. By adding HCl, the pH of the reaction mixture can be lowered, making the environment more acidic. Conversely, the addition of a base such as sodium hydroxide (NaOH) can raise the pH of the reaction mixture.
(3) To remove impurities or byproducts: In some reactions, the addition of HCl can help to remove impurities or byproducts that may interfere with the desired reaction. For example, HCl can be used to remove metal oxides or hydroxides from a reaction mixture.
Learn more about protons here:
https://brainly.com/question/1252435
#SPJ1
A stock solution of HNO3 is prepared and found to contain 14.2 M of HNO3. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, the concentration of the diluted solution is ________ M.
Answers
Answer:
First convert volume given into same unit.
Therefore 1000 mL =1L
1000mL=1L
25.0mL=?
(25.0×1)÷1000=0.025L
but using the equation;M1×V1=M2×V2
M1=14.2M
V1=0.025L
M2=?
V2=0.5L
Therefore;. 14.2×0.025=M2×0.5
M2=(14.2×0.025)÷0.5
M2=0.71M.
A stock solution of HNO3 is prepared and found to contain 14.2 M of HNO3. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, the concentration of the diluted solution is 0.71M.
What is the stock solution ?To make a stock solution, weigh out the proper amount of a pure solid or measure out the proper amount of a pure liquid, put it in the right flask, and then dilute it to the desired volume. Depending on the intended concentration unit, many methods can be used to measure the reagent.
First we convert volume
Then 1000 mL = 1L
1000mL= 1L
25.0mL = ?
( 25.0 × 1 ) / 1000
= 0.025L
by using the equation;
M1 × V1 = M2 × V2
M1 = 14.2M
V1 = 0.025L
M2 = ?
V2 = 0.5L
14.2 × 0.025 = M2 × 0.5
M2 = (14.2 × 0.025 ) ÷ 0.5
M2 = 0.71M.
Thus, A stock solution of HNO3 is prepared and found to contain 14.2 M of HNO3. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, the concentration of the diluted solution is 0.71M.
To learn more about stock solution, follow the link;
https://brainly.com/question/17018950
#SPJ5
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2
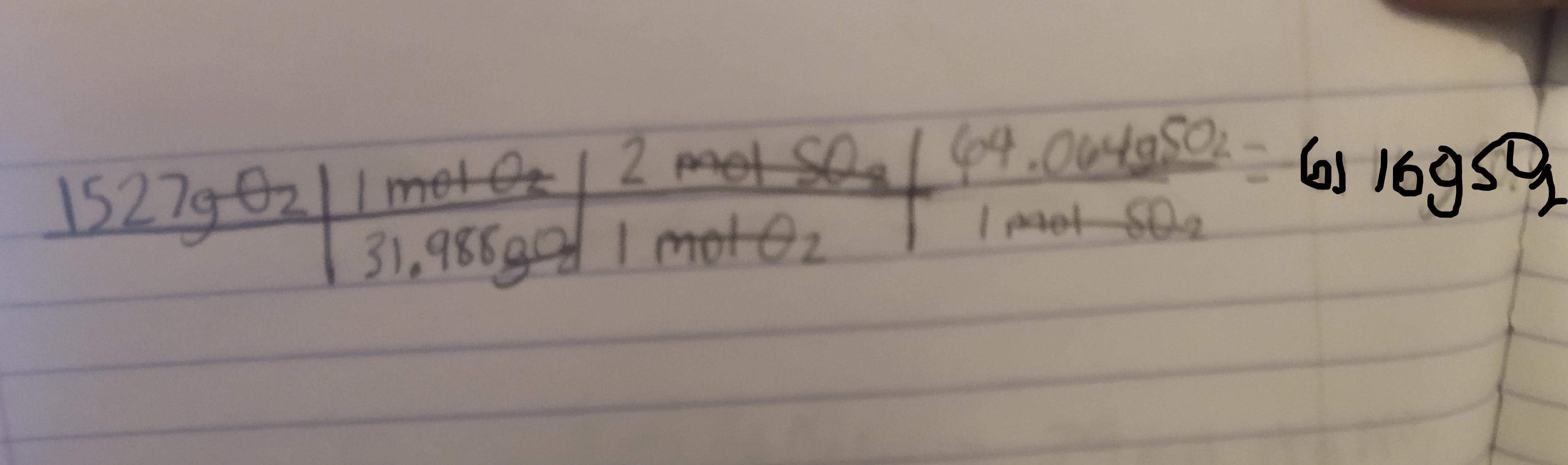
Calculate the pH of the following:
1. [H+] = 1 x 10-7 M
2. [OH-] = 1 x 10-3 M
3. [H+] = 1 x 10-2 M
4. [H+] = 1 x 10-10 M
5. [OH-] = 1 x 10-8 M
Answers
The pH can be defined as the negative logarithm of the hydrogen ion concentration of the solution.
What is the pH?What we call the pH can be defined as the negative logarithm of the hydrogen ion concentration of the solution. We are aware that we can use the relation [H+] [OH-] = 1 * 10^-14 to handle the enormity of this problem.
Now, let us go about solving the problems;
1. pH = -log(1 x 10-7) = 7
2. [H+]= 1 * 10^-14/ 1 x 10^-3
pH = -log( 1 * 10^-11)
pH = 11
3. pH = -log( 1 x 10^-2)
pH = 2
4. pH = -log( 1 x 10^-10)
pH = 10
5. [H+]= 1 * 10^-14/ 1 x 10^-8
[H+]= 1 * 10^-6
pH = 6
Learn more about pH:https://brainly.com/question/1528974
#SPJ1
A compound contains 0.5 mol Na, 0.5 mol N, and 10 mol H. The empirical formula of the
compound is -
Answers
Answer:
NaNH₂₀
Explanation:
0.5 mol Na, 0.5 mol N, and 10 mol H
To obtain the empirical formulae, we find the mole ratio between the elements and this is done by dividing all through by the smallest mol (0.5)
Na = 0.5 / 0.5 = 1
N = 0.5 / 0.5 = 1
H = 10 / 0.5 = 20
The mole ratio is used to write the empirical formulae. It is given as;
NaNH₂₀
What volume of silver metal will have a mass of exactly 2500.0 g? The density of silver is 20.5 g/cm3.
Answers
Answer: 122 cm^3
Explanation:
2500.0 g * 1 cm^3/20.5 g=
2500.0 * 1/20.5=
2500.0/20.5=122 cm^3
classifying as acidic, basic, or neutral
Answers
Acidity, basicity, and neutrality are terms used to describe the properties of a solution. A solution can be classified as acidic, basic, or neutral based on its pH value, which is a measure of the concentration of hydrogen ions (H+) in the solution.
What is pH?
It is defined as the negative logarithm (base 10) of the concentration of hydrogen ions (H+) in the solution. The pH scale ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most basic (alkaline).
Acids are substances that can donate hydrogen ions (H+) to a solution, thereby increasing the concentration of H+ ions. Examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).
If the pH of a solution is greater than 7, it is considered basic or alkaline. The higher the pH, the more basic the solution is. Bases are substances that can accept hydrogen ions (H+) from a solution, thereby reducing the concentration of H+ ions. Examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
If the pH of a solution is exactly 7, it is considered neutral. This means that the concentration of H+ ions is equal to the concentration of hydroxide ions (OH-) in the solution. Water is an example of a neutral solution, as it has a pH of 7.
Learn more about pH from given link
https://brainly.com/question/172153
#SPJ1
The electrical current in a circuit is measured in which of the following units
Answers
Answer:
joules
Explanation:
i think
Explanation:
The SI unit of electric current is the ampere, or amp, which is the flow of electric charge across a surface at the rate of one coulomb per second. The ampere (symbol: A) is an SI base unit Electric current is measured using a device called an ammeter.
A chipmunk has a mass of 0.7 kg. What is its weight? (Acceleration due to gravity on Earth is 9.8 m/s2).
Answers
Answer:
W=ma
=0.7×9.8
=6.86N
....................
PLEASE GIVE BRAINLIEST
Which statement best describes why water is a polar molecule?
a. Water has a slightly negative oxygen atom and slightly positive hydrogen atoms.
b. Water has nonpolar bonds that cancel each other out due to similar electronegativity values.
c. Water has bonds where electrons are equally shared between oxygen and hydrogen atoms.
d. Water has a slightly positive oxygen atom and slightly negative hydrogen atoms.
Answers
Hydrogen atoms are slightly positive and oxygen atoms are slightly negative in water best describes water as polar molecule. Option A is correct.
Due to the molecule's bent shape, water (H₂O) is polar. The shape implies the greater part of the negative charge from the oxygen on side of the particle and the positive charge of the hydrogen atom is on the opposite side of the atom
Two properties of water that outcome from the polar holding between its atoms are attachment and bond. These properties make water atoms 'adhere' to one another and to be drawn to other polar particles.
A polar particle is typically shaped when the one finish of the particle is said to have more certain charges and though the far edge of the atom has negative charges, making an electrical shaft.
Learn more about Polar molecule:
brainly.com/question/17118815
#SPJ1
Which statement describes an intensive property of matter?
OIt is the same for every sample of a single substance.
OIt depends on how a substance was formed.
It is the same for every sample of every substance.
OIt depends on the amount of substance present.
27
Answers
It is the same for every sample of every substance present describes an intensive property of matter.
The correct option is C.
What is the meaning of intensive property?An intense property is a greater stability of a system that is independent of the system's size or the volume of its constituent elements. Based on the definitions, internal energy, volume, or density are extensive properties while pressure, temperature, and densities are intensive properties.
Why density is a intensive property?Because of the small range of densities present among the samples, density is an intense attribute. Concentrations were roughly the same regardless of the beginning mass. The data show that density is an intense attribute of matter because it is independent of the amount of substance present.
To know more about Intensive property visit:
https://brainly.com/question/13733851
#SPJ13
The complete question is
Which statement describes an intensive property of matter?
A-It is the same for every sample of a single substance.
B-It depends on how a substance was formed.
C-It is the same for every sample of every substance.
D-It depends on the amount of substance present.
If a package of nuts weighs 41.3 what is the massif package express in milligram

Answers
Answer:
1170855 mg
Explanation:
To identify a diatomic gas ( X2 ), a researcher carried out the following experiment: She weighed an empty 6.4- L bulb, then filled it with the gas at 1.30 atm and 27.0 ∘C and weighed it again. The difference in mass was 9.5 g . Identify the gas.
Answers
The diatomic gas ( X2 ) is N₂ dinitrogen.
Dinitrogen is a chemical compound fashioned from the covalent bonding of two nitrogen atoms. it's far a colorless, odorless gas at room temperature and pressure, which makes up about seventy-eight % of the Earth's environment.
Diatomic gas is a chemical compound formed from the covalent bonding of two nitrogen atoms. it's miles drab, odorless gasoline at room temperature and stress, which makes up about seventy eight % of the Earth's surroundings.
Volume = 6.4 L
Pressure = 1.3 atm
Temperature = 25 C = 298 K
R = 0.08206 L.atm/mol.K
P * V = n * R * T
1.3 atm * 6.4 L = n * 0.08206 L.atm/mol.K * 298 K
n = 0.34 moles
difference of mass is the mass of gas = 9.5 g
Molar mass = Mass / No. of moles = 9.5 g / 0.34 moles = 27.9 g/mol
diatomic gas with molar mass 28 g/mol is N2
Hence the diatomic molecule is N2
Learn more about dinitrogen here:-https://brainly.com/question/11651796
#SPJ9
The wavelength of infrared light is 1000 nm. What is the frequency in Hz? c=3.00x10^8m/s
ans choices are
3.0x10^14
4.0x10^15
1.0x10^13
2.0x10^14
4.0x10^14
Answers
\(\\ \tt\Rrightarrow \nu=\dfrac{c}{\lambda}\)
v is frequencylambda is wavelength\(\\ \tt\Rrightarrow \nu=\dfrac{3\times 10^8}{1000\times 10^{-9}}\)
\(\\ \tt\Rrightarrow \nu=0.003\times 10^{17}s^{-1}\)
\(\\ \tt\Rrightarrow \nu=3\times 10^{14}Hz\)
A person on a diet loses 15 lb in 3.5 months. Calculate the average weight loss in milligrams per second (mg/s).
Answers
The average weight loss is 0.739 mg/s.
The total weight lost by the individual = 15 lb
To convert Ib to mg
1 Ib = 453592 mg
15 lb = 15 lb * 453592 mg/ 1 Ib
= 6803880 mg
The time taken for the weight loss = 3.5 months
Number of seconds in 3.5 months = 9.198 * 10^6 seconds
Hence;
Average weight loss in milligrams per second (mg/s);
Weight lost/ time taken
6803880 mg/9.198 * 10^6 seconds
= 0.739 mg/s
Learn more: https://brainly.com/question/4736731
charles law . who can please help me with these two problems asap ?

Answers
Answer:
k
Explanation:
Use dimensional analysis to compute what volume of titanium (in cm3) would have a mass of 68.9186 grams. DO not include units in your answer, just the numeric part, and report your result to the correct number of significant figures.
Answers
The volume of titanium would have a mass of 68.9186 grams is 15.180 cm³.
What is volume ?The term volume is defined as the space occupied within the boundaries of an object in three-dimensional space. It is also known as the capacity of the object. Two examples of cubic units are cm3 or in3.
Given:
Density = 4.540 gm/cm3
mass = 68.9186 grams
Volume = ?
As we know the formula of density = mass / volume
Therefore, volume = mass / density
volume = 68.9186 gm / 4.540 gm/cm3
volume = 15.1803 cm³
Thus, the volume of titanium would have a mass of 68.9186 grams is 15.180 cm³.
To learn more about the volume, follow the link;
https://brainly.com/question/1578538
#SPJ1
Question 2 of 10
Which chemical equation is balanced?
A. CO₂ + H₂O → H₂CO₂
B. K+ H₂O → K₂O + H₂
C. CaO2 + HCl → CaCl₂ + H₂O
OD. MgO + 2HCl → MgCl₂ + H₂O
Answers
The equation MgO + 2HCl → MgCl₂ + H₂O is a balanced equation.
A balanced chemical equation contains equal number of atoms on both sides of the equation.The equation is MgO + 2HCl → MgCl₂ + H₂O which contains 1 atom of Mg, 2 atoms of chlorine, 2 atoms of chlorine, 1 atom of oxygen on both reactants and products sides.So the equation is balanced.An equation for a chemical reaction is said to be balanced if both the reactants and the products have the same number of atoms and total charge for each component of the reaction. In other words, both sides of the reaction have equal amounts of mass and charge.It follows law of conservation of mass.Mass is neither created nor destroyed rather it can be transferred from one form to another.Learn more about balanced equation at:
brainly.com/question/2829417
#SPJ9
What is the coefficient for sodium chloride when this equation is balanced?
Answers
Answer:
To resolve this, we need to place the coefficient “2” in front of the sodium in the reactant, to give the equation shown below. 2 Na (s) + Cl 2 (g) → 2 NaCl (s) In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in the products; the equation is now balanced.
Explanation:
A 105 L container holds 32 mol of gas. How many moles of gas will
there be if 40 L of gas were removed?
Pls help!
Answers
vitamin A in chemistry
Answers
Answer:
\(\sf\fbox\red{Answer:-}\)
Vitamin A is a fat-soluble vitamin and an essential nutrient for humans. It is a group of organic compounds that includes retinol, retinal (also known as retinaldehyde), retinoic acid, and several provitamin A carotenoids (most notably beta-carotene (β-carotene).
\(\small\fbox{\blue{\underline{mαrk \; mє \; вrαínlíєѕt \; plєαѕє ♥}}}\)
Write a balanced nuclear equation for the beta decay of 234/90Th

Answers
The balanced nuclear equation for the beta decay of 234/90Th can be represented as follows: ^234/90Th --> ^234/91Pa + e^0/-1β
In this equation, the nucleus of thorium-234 (234/90Th) undergoes beta decay. During beta decay, a neutron within the nucleus is converted into a proton, resulting in the emission of an electron (beta particle). As a result, the thorium-234 nucleus is transformed into protactinium-234 (234/91Pa) by gaining one proton.
The beta particle emitted during the decay process is represented as e^0/-1β, where the superscript 0 denotes that the electron has no charge (neutral), and the subscript -1 indicates that the electron carries a negative charge of -1.
It is important to note that in a nuclear equation, the total atomic mass and atomic number on both sides of the equation must be equal to maintain a balanced equation and conserve mass and charge.
For more such questions on balanced nuclear equation visit:
https://brainly.com/question/31505524
#SPJ8
Please Help! Chemistry questions below!

Answers
So
Moles of Oxygen:-
44.7/321.396mol3 mol of O_2 produces 2 mol SO_2
1 mol of ao_2 produces 2/3=0.6mol So_2
1.396 mol produces 0.8376mol SO_2
Mass of SO_2
0.8376(64)53.6g