Answers
The number of mole of CaCO₃ in antacid tablet containing 0.512 g of CaCO₃ is 5.12×10⁻³ mole
Description of moleThe mole of a substance is related to it's mass and molar mass according to the following equation:
Mole = mass / molar mass
How to determine the mole of CaCO₃From the question given above, the following data were obtained:
Mass of CaCO₃ = 0.512 gMolar mass of CaCO₃ = 40 + 12 + (3 × 16) = 40 + 12 + 48 = 100 g/mol Mole of CaCO₃ =?The number of mole in 0.512 g of CaCO₃ can be obtained as follow:
Mole = mass / molar mass
Mole of CaCO₃ = 0.512 / 100
Mole of CaCO₃ = 5.12×10⁻³ mole
Thus, 5.12×10⁻³ mole of CaCO₃ is present in the antacid tablet
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
Related Questions
I don’t understand how to do this
Answers
Answer:
do what?????
Explanation:
How can you identify the gas produced in a chemical reaction without observing the atoms and molecules directly?
Answers
To identify the gas produced in a chemical reaction without observing the atoms and molecules directly through the characteristics of the gas.
Chemical reaction is defined as the process by which substances (known as reactants) combine together with the formation of bonds to form products.
There are some signs that shows that chemical reaction has occurred. They include:
Color Change Change of temperatureGiving off gas (Evolution of gas)Therefore, the production of gas from a chemical reaction shows that a reaction has occurred.
These gases can be identified without observing the molecules through that characteristics of the gas. This include:
the smell of the gas: For example when a gas is given off, and it smells like urine, it's likely to be ammonia gas. color change that occurs during the chemical reaction can show the type of gas that was given off. For example when lime water turns into milky color, it shows the presence of carbondioxide gas.Learn more here:
https://brainly.com/question/15740593
compared to sodium, sulfur is more-
A. conductive
B. shiny
C. dull
D. malleable
Answers
WHAT TYPE OF REACTION IS HAPPENING WITH NI2O3 (s) Ni (s) + O2 (g)
Answers
Answer:
Decomposition.
Explanation:
Hello!
In this case, since we can find four types of reactions, combination, decomposition, single displacement and double displacement, for the reaction:
2NI2O3 (s) --> 4Ni (s) + 3O2 (g)
We can evidence that the reactant (only one) is separated into the elements forming it; therefore, we infer this is a decomposition reaction.
Best regards!
What is the pH of a solution the hydroide concentration of 1 X 10.5?
Answers
Answer:
Explanation:
Therefore, the pH of the HCl solution is 5.
Where are the most gases located on the periodic table?
Answers
Answer:
They are mostly located in group 18 (column 18), or far right
lndicate the ionisation of the following acids,tetraoxosulphate (vi)acid,trioxonitrat
e(v)acid,ethanoic acid.
Answers
The ionization of the following acids can be represented as:
Tetraoxosulphate (VI) Acid (\(H_{2}SO_{4}\)) ionizes as H+ and SO4^2- ions.
Trioxonitrate (V) Acid (\(HNO_{3}\)) ionizes as H+ and \(NO_{3-}\) ions.
Ethanoic Acid (\(CH_{3}COOH\)) ionizes as H+ and \(CH_{3}COO^{-}\) ions.
Tetraoxosulphate (VI) Acid, also known as sulfuric acid (\(H_{2}SO_{4}\)), ionizes as follows:
\(H_{2}SO_{4}\) → \(H+\) + \(SO_{4}^{2-}\)
In this reaction, sulfuric acid donates two hydrogen ions (H+) to the solution, forming sulfate ions (\(SO_{4}^{2-}\)).
Trioxonitrate (V) Acid, commonly known as nitric acid (\(HNO_{3}\)), ionizes as follows:
\(HNO_{3}\) → \(H+_{}\) + \(NO_{3-}\)
Nitric acid dissociates to release one hydrogen ion (\(H+\)) and a nitrate ion (\(NO_{3-}\)).
Ethanoic Acid, also known as acetic acid (\(CH_{3}COOH\)), ionizes as follows:
\(CH_{3}COOH\) → H+ + \(CH_{3}COO^{-}\)
Acetic acid donates a hydrogen ion (H+) to the solution, forming an acetate ion (\(CH_{3}COO^{-}\)).
In all cases, the acids dissociate in water, producing hydrogen ions (H+) as positively charged ions and their corresponding anions. The hydrogen ions are responsible for the acidic properties of these substances, while the anions contribute to the overall charge balance in the solution. The ionization of acids allows them to conduct electricity in aqueous solutions and react with other substances.
The question was incomplete. find the full content below:
Indicate the ionization of the following acids,
Tetraoxosulphate (VI) Acid
Trioxonitrate (V) Acid
Ethanoic Acid.
Know more about ionization here:
https://brainly.com/question/30831422
#SPJ8
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
A 16.45 gram sample of iron is heated in the presence of excess oxygen. A metal oxide is formed with a mass of 21.16 g. Determine the empirical formula of the metal oxide
Answers
One mole of iron is equal to one mole of oxygen. As a result, FeO is the empirical formula for the metal oxide.
What is rust's empirical formula?Rust has the chemical formula Fe2O3, also known as ferric oxide or iron oxide. The result is a chain of chemical processes that can be summed up as follows: The formula for rusting iron is 4Fe + 3O2 + 6H2O 4Fe (OH) 3. Water and oxygen are both necessary for the rusting process.
By deducting the mass of the iron from the overall mass of the metal oxide, we can determine the mass of oxygen that combined with the iron:
mass of oxygen = mass of metal oxide - mass of iron
mass of oxygen = 21.16 g - 16.45 g
mass of oxygen = 4.71 g
Then, using their respective molar masses, we can convert the masses of iron and oxygen to moles:
moles of iron = 16.45 g / 55.85 g/mol = 0.294 mol
moles of oxygen = 4.71 g / 16.00 g/mol = 0.294 mol
To know more about empirical formula visit:-
https://brainly.com/question/14044066
#SPJ1
The sun causes a greenhouse effect on Earth. How is Earth impacted by the greenhouse effect?(1 point)
1. It allows for oceans to warm up quicker than land.
2. It creates the different seasons that occur on Earth.
3. It allows for people to inhabit Earth.
4. It is the source of day and night on Earth.
Answers
Answer: Sunlight passes through the atmosphere and warms the Earth's surface. ... As more greenhouse gases are emitted into the atmosphere, heat that would normally be radiated into space is trapped within the Earth's atmosphere, causing the Earth's temperature to increase.
Explanation: This keeps well life on earth so the answer is (3)
:)
The sun causes a greenhouse effect on Earth. Earth is impacted by the greenhouse effect due to it allows for people to inhabit Earth. Option 3 is correct.
The greenhouse effect is a natural phenomenon that plays a critical role in maintaining Earth's temperature within a range suitable for sustaining life. It occurs when certain gases in the Earth's atmosphere, such as carbon dioxide and water vapor, absorb and re-radiate infrared radiation from the sun, effectively trapping heat in the atmosphere. This trapped heat warms the planet's surface and prevents it from becoming too cold.
Without the greenhouse effect, Earth's average temperature would be much lower, making it inhospitable for humans and many other species. The presence of liquid water, which is essential for life as we know it, would be greatly compromised. The greenhouse effect, while natural, has been enhanced by human activities such as burning fossil fuels, leading to an increased concentration of greenhouse gases and contributing to global warming and climate change.
Hence, 3. is the correct option.
To know more about greenhouse effect here
https://brainly.com/question/31595505
#SPJ3
How many significant figures
are in this number?
23,479
Answers
Local winds can be caused by______heating of the earth's surface.
equal
ground
uneven
super
Answers
how do x rays use electromagnetic waves?
Answers
Answer:
X-rays are a form of electromagnetic radiation, similar to visible light. Unlike light, however, x-rays have higher energy and can pass through most objects, including the body. Medical x-rays are used to generate images of tissues and structures inside the body.
Explanation:
Explanation:Los rayos X son un tipo de radiación llamada ondas electromagnéticas. Las imágenes de rayos X muestran el interior de su cuerpo en diferentes tonos de blanco y negro. Esto es debido a que diferentes tejidos absorben diferentes cantidades de radiación.
El uso más común de los rayos X es para ver fracturas (huesos rotos), pero también se utilizan para otros usos. Por ejemplo, las radiografías de tórax pueden detectar neumonía. Las mamografías utilizan rayos X para detectar el cáncer de mama.
Prepare 0.25M Cl in 250ml from Bacl2.2H2O
Answers
We need to weigh 15.27 g of \(BaCl_{2}.2H_{2}O\) and dissolve it in water to make a 0.25M Cl solution in 250ml.
To prepare 0.25M Cl in 250ml from \(BaCl_{2}.2H_{2}O\) we first need to calculate the required amount of \(BaCl_{2}.2H_{2}O\).
The molecular weight of \(BaCl_{2}.2H_{2}O\) is 244.26 g/mol.
The equation for the dissociation of \(BaCl_{2}\) in water is:
\(BaCl_{2}\)(s) → \(Ba^{2+}\) (aq) + 2Cl- (aq)
To make 250 ml of 0.25M Cl solution, we need to calculate the amount of\(BaCl_{2}.2H_{2}O\)required to produce 0.25 moles of Cl.
0.25 moles Cl × 1 mole \(BaCl_{2}\)/ 2 moles Cl × 244.26 g/mol \(BaCl_{2}\)= 15.27 g \(BaCl_{2}.2H_{2}O\)
Therefore, we need to weigh 15.27 g of \(BaCl_{2}.2H_{2}O\) and dissolve it in water to make a 0.25M Cl solution in 250ml.
Learn more about dissociation, here:
https://brainly.com/question/13363803
#SPJ1
18. Underline the significant zeros in the following numbers
a. 5.08
b. 508
c. 5.080 x 10³
d. 0.05080
Answers
According to significant figures, the significant zeros in the following numbers are 5.08 b. 508 c. 5.080 x 10³ d. 0.05080.
What are significant figures?Significant figures are used for establishment of a number which is presented in the form of digits. These digits give a meaningful representation to the numbers.
The significant figures are the significant digits which convey the meaning according to the accuracy. These provide precision to the numbers and hence are called as significant numbers.There are rules for counting significant figures which are as follows:
1)All non-zero digits are significant .
2)All zeroes which occur between non-zero digits are significant.
3)All zeroes to the left and right of a non-zero digit are not significant.
4) All zeroes on right of decimal are significant if a non-zero number follows them.
5)All zeroes on right side of non-zero digit are significant.
Learn more about significant figures,here:
https://brainly.com/question/29153641
#SPJ1
A piston–cylinder device initially contains 0.33-kg steam at 3.5 MPa, superheated by 107.4oC. Now the steam loses heat to the surroundings and the piston moves down, hitting a set of stops at which point the cylinder contains saturated liquid water. The cooling continues until the cylinder contains water at 200oC. What is the amount of heat transfer when the piston first hits the stops and the total heat transfer
Answers
Both the initial heat transfer when the piston initially contacts the stops and the overall heat transfer throughout the procedure are -11,172 kJ.
How do you figure out how much heat is transferred overall and when the piston first strikes the stops?h1 = 3279.1 kJ/kg
h2 = 751.6 kJ/kg
Q = ΔU = m(u2 - u1) (u2 - u1)
where m is the steam's mass.
We may use the ideal gas law to determine the mass of the steam:
PV = mRT
PV/(RT) = (3.5 MPa) (0.33 m3)/(0.287 kJ/kg-K) (380.4 K) = 4.47 kg for the formula m.
We can now determine the rate of heat transfer:
Q is equal to m(u2 - u1) = (4.47 kg)(751.6 kJ/kg - 3279.1 kJ/kg) = -11,172 kJ
Heat is leaving the system, as indicated by the negative sign.
To learn more about piston-cylinder device visit:
brainly.com/question/22969319
#SPJ9
A drawing.Short description, A drawing.,Long description,
The drawing shows a wave of water moving to the right in a container. As the wave moves, the distance between the peak and trough of the wave gets shorter, but the distance between the peaks of the waves remains the same.
Question
What does the drawing show about the energy of the wave as it moves?
Answer options with 4 options
A.
The energy of the wave increases as shown by the increasing frequency.
B.
The energy of the wave decreases as shown by the decreasing amplitude.
C.
The energy of the wave remains the same as shown by the same wavelength.
D.
The energy of the wave regularly increases and decreases as shown by the peaks and troughs.
the picture is this:

Answers
Answer:
wow 15 points is alot
Explanation:
If [H3O^ + ]=1.7*10^ -8 M what is the pOH of the solution?
Answers
Answer: 6.23
Explanation:
1) solve for pH
pH=-log (H3O+) = - log 1.7 X 10^-8 =7.77
2) now do 14-pH = 14 -7.77=6.23
PLEASE HELP "4s2 is the amount of energy in the atom"
Using the terms energy level, sub level, and number of electrons explain WHY this misconception is incorrect and what "4", "s" and "2" represent in electron configurations.
Answers
The two electrons are found in the N shell of the atom and in a spherically symmetrical orbital.
What is electron configurations?The electron configuration refers to the manner in which electrons are arranged in an atom. The electron in an atom are arranged in shells and subshells. The shells are shown by the principal quantum number which the subshells are shown by the orbital quantum number.
The orbital where the electron is found has a direct corroboration to the energy of the electron. The more radially outwards from the nucleus the orbital is as evidenced by the orbital quantum number, the higher the energy of the electron.
Now the designation"4s^2" implies that the two electrons are found in the N shell of the atom and in a spherically symmetrical orbital.
Learn more about electron configuration:https://brainly.com/question/14283892
#SPJ1
What’s a carbohydrate?
A. A hormone
B. A cholesterol molecule
C. An enzyme
D. A sugar molecule
Answers
How to balance NH3+NO=N2+H2O
Answers
Explanation:
NH₃+NO=N₂+H₂O;
NH₃+NO=N₂+6H₂O;
NH₃+6NO=N₂+6H₂O;
4NH₃+6NO=N₂+6H₂O;
4NH₃+6NO=5N₂+6H₂O.
A helium balloon with an internal pressure of 1.00 atm and a volume of 4.50 L at 20.00 C is released. What volume will the balloon occupy at an altitude where the pressure is 0.600 atm and the temperature is -20.00 C?
Answers
The volume the balloon will occupy at an altitude where the pressure is 0.600 atm and the temperature is -20.00 C is 6.15 L.
The ideal gas law can be used to solve the given problem, which is as follows:P1V1/T1 = P2V2/T2 Where:P1 is the initial pressure (1.00 atm)P2 is the final pressure (0.600 atm)V1 is the initial volume (4.50 L)V2 is the final volume (what we want to find)T1 is the initial temperature (20.00 C = 293.15 K)T2 is the final temperature (-20.00 C = 253.15 K)The problem does not provide the mass or number of moles of helium gas, but these quantities are not required to solve the problem as they cancel out in the calculation.The problem also does not mention any change in altitude, so we can assume that the balloon rises to an altitude where the pressure and temperature are lower.We can rearrange the equation above to solve for V2, which gives:V2 = (P1V1T2)/(P2T1) Substituting the given values gives:V2 = (1.00 atm × 4.50 L × 253.15 K)/(0.600 atm × 293.15 K)V2 = 6.15 L.
for such more questions on volume
https://brainly.com/question/29796637
#SPJ8
i hope y’all understand lolz

Answers
Answer:
protons are red neutrons are green electrons are yellow
Explanation:
TIE FIGHTERS GO ZOOOOOM
I’m not sure and I’m kind of confused can anyone help?
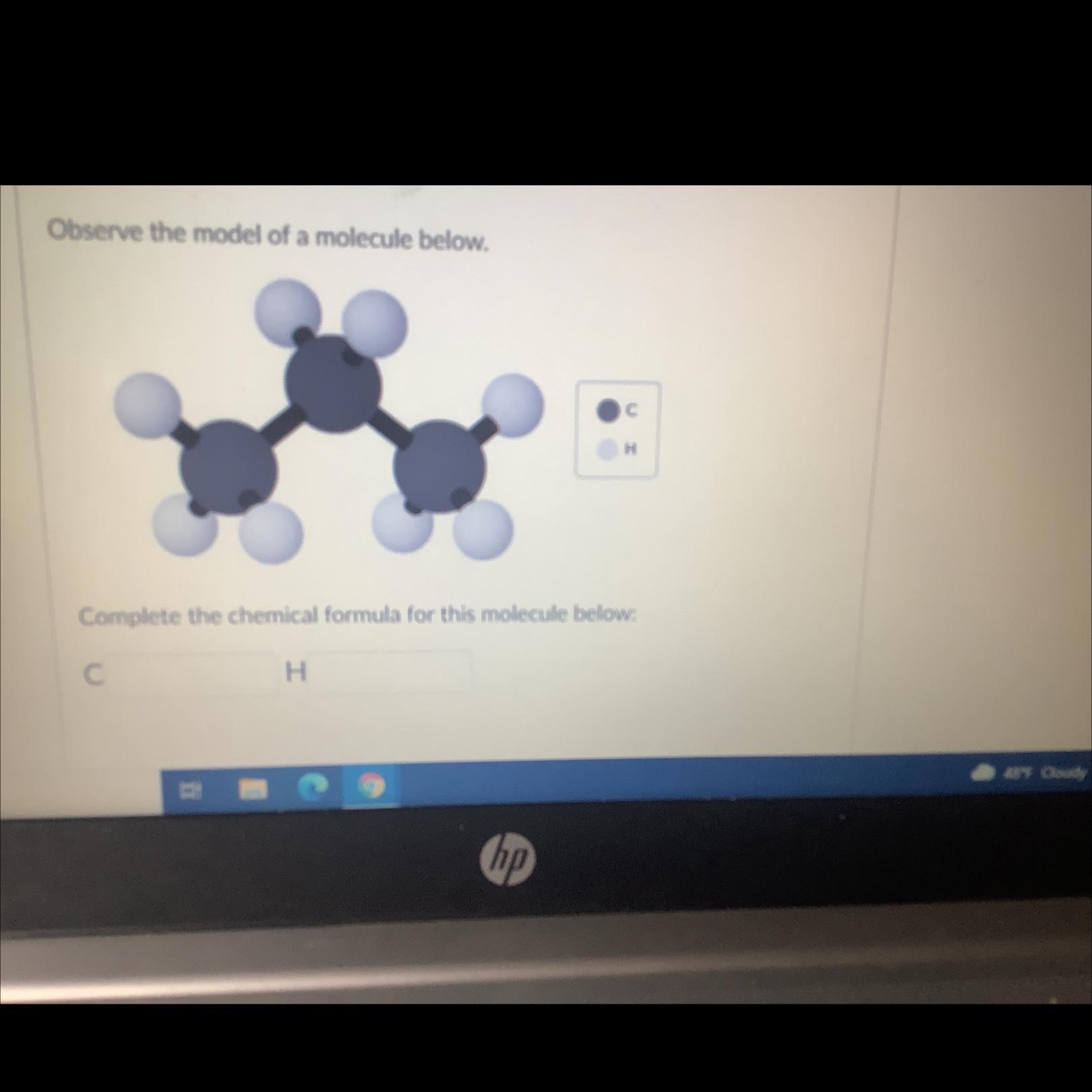
Answers
We will reconstruct the model in the following manner :
From the above diagram we can see that :
• number of Carbon atom = 3
• number of hydrogen atom = 8
• rewrite this in an alphabetical order, you get :
\(\begin{gathered} C_3H_8\text{ } \\ \Rightarrow Propane\text{ } \end{gathered}\)the molecule has a chemical formula = C3H8
A 600. mL beaker has an inner diameter of 77.0 mm. What is the vertical distance between the 100. mL marks on the side of the beaker
Answers
Answer:
\(h=12.9cm\)
Explanation:
Hello!
In this case, since we can consider the beaker until the 100-mL mark as a cylinder, we can use the following equation to relate its diameter, vertical distance or height and volume:
\(V=\pi h\frac{d^2}{4}\)
Thus, since we know the diameter, volume (which is equivalent to 600 cm³) and π, we can plug in to obtain:
\(600cm^3=\pi *h*\frac{(77.0mm)^2}{4}\)
It means it is necessary to take the mm to cm and solve for h:
\(h=\frac{600cm^3}{\pi*\frac{(7.70cm)^2}{4}} \\\\h=12.9cm\)
Best regards!
The distance between each 100 mL mark is 2.15 cm.
The volume of a cylinder is obtained using the formula;
V = πr^2h
Now, we have the following information;
Volume of the cylinder = 600. mL or 600 cm^3
Diameter of the cylinder = 77 mm or 7.7 cm
Radius of the cylinder = 7.7/2 = 3.85 cm
Height of the cylinder = h
Hence;
600 = 3.142 × ( 3.85 )^2 × h
h = 600/3.142 × ( 3.85 )^2
h = 12.88 cm
There are six 100 mL marks on the beaker, the distance between each 100 mL mark = 12.88 cm/6 = 2.15 cm
Learn more about volume: https://brainly.com/question/12748872
Calculate how many grams of BeCl2 are required to produce 0.52 grams of MnCl2
Answers
Answer:
65.0cp
Explanation:
What was the molecular geometry for SC16?
Answers
Answer:
I'm assuming this is for the SCl₆ compound and not SC₁₆? If so, the molecular geometry is octahedral.
Explanation:
There are 6 regions and zero lone pairs surrounding the central atom (S).
To find the order of a reaction with respect to one reactant, you will monitor the as the of . is changed.
Answers
The order of reaction is defined as the power to which the concentration of the reactants are raised in the rate equation of the reaction.
The order of reaction can be used to determine how a particular reactant affects the reaction. In order to find the order of a reaction with respect to a particular reactant, the concentration of the reactant is changed while keeping the concentration of other reactants constant. The rate of reaction is then measured and compared with the rate of reaction when the concentration of the reactant is not changed.The order of reaction with respect to a reactant can be determined using the following method:First, select a reactant whose order needs to be determined and change its concentration while keeping the concentration of other reactants constant. For example, if we want to find the order of reaction with respect to reactant A, we will change the concentration of A and keep the concentration of reactant B constant.Second, measure the rate of reaction at different concentrations of the reactant A. The rate of reaction can be measured by any suitable method such as change in color, pH, or by measuring the amount of product formed with time. A graph is plotted with rate of reaction on the y-axis and concentration of reactant A on the x-axis. The graph should be a straight line.Third, if the graph is a straight line passing through the origin, the order of reaction with respect to reactant A is one. If the graph is a straight line but does not pass through the origin, the order of reaction with respect to reactant A is two. If the graph is not a straight line, the order of reaction with respect to reactant A is either zero or fractional.For such more question on concentration
https://brainly.com/question/17206790
#SPJ8
Naturally occurring silicon consists of three isotopes with the following isotopic masses and abundances. Isotope Isotopic mass (u) Abundance (%) 2828 Si 27.976926532727.9769265327 92.229792.2297 2929 Si 28.9764947228.97649472 4.68324.6832 3030 Si 29.9737702229.97377022 3.08723.0872 Calculate the average atomic mass of naturally occurring silicon to at least four significant figures.
Answers
The question is incomplete, the complete question is;
Silicon has three naturally occurring isotopes with thefollowing masses and natural abundances:
Isotope Mass ({\rm amu}) Abundance (%)
{\rm Si}-28 27.9769 92.2
{\rm Si}-29 28.9765 4.67
{\rm Si}-30 29.9737 3.10
Calculate the atomic mass of silicon.
Answer:
28.09 amu (to four significant figures)
Explanation:
Given that;
Isotope Mass Abundance (%)
Si-28 27.9769 92.2
Si-29 28.9765 4.67
Si-30 29.9737 3.10
So we now have;
Relative atomic mass of silicon;
(27.9769 × 0.9218) + (28.9765 × 0.0471) + (29.9738 × 0.0312)
25.789 + 1.365 + 0.9351 = 28.09 amu (to four significant figures)
What are the charges of the ions in an ionic compound containing cobalt(III) and fluoride ions?
Write the formula for the compound.
Answers
The charge on the ions in an ionic compound containing cobalt(III) and fluoride ions is Co³⁺ and F⁻¹ and the formula of the compound is CoF₃.
Ionic compounds are a type of chemical compound where the oppositely-charged ions of a metal and a nonmetal are attracted to each other to form an ionic bond.
The compound formed from the bonded ions will have very different properties from the elements that make up the compound.
While atoms are neutral because they have an equal number of protons and electrons, ions have a net charge and result when an atom loses or gains electrons.
Learn more about Ionic compound, here:
https://brainly.com/question/3222171
#SPJ1