How many moles are in 15.0 g Aluminum
Answers
The number of moles in 15.0 g of Aluminum having an atomic mass of 27 g/mol is 0.556 mol.
Now,
we know that,
number of moles = given mass/molar mass
And, the molar mass of Aluminum is 27 units.
so,
number of moles = 15.0 / 27
= 0.556
hence , 0.556 moles are in 15.0 g of Aluminum.
Learn more about Moles at :
https://brainly.com/question/1427604
Related Questions
Which of the following is a product of the
photoelectric effect?
A. a neutral atom
B. ejection of a proton
C. ejection of a neutron
D. an ion
Answers
Photoelectric effect is the ejection of electrons from the metal surfaces, when light hos on it. The ejection of electrons makes the surface metal atoms to produce ions. Hence, D is correct.
What is photoelectric effect ?The phenomenon of ejection of electrons from the surface of a metal when light of a suitable frequency falls on it is called photoelectric effect. Upon striking the surface of metals , light can set up an electric current which current which corresponds to the flow of electrons. This phenomenon is discovered by Einstein
For instance, visible light can cause ejection of electrons from the surface of metal like cesium which has low ionization enthalpy. This phenomenon is related to the observation that if light is considered as particles.
The particles of light is then called as photoelectrons or photons. Photoelectric effect thus, produce the metal ions by ejecting electrons from the atom.
Find more on photoelectric effect:
brainly.com/question/26465043
#SPJ9
An atom has mass number 23 and atomic number 11.
a) How many electrons are revolving around the nucleus?
b) How many electron shells are there in the atom?
Answers
A): The closest orbital to the nucleus, called the 1s orbital, can hold up to two electrons. This orbital is equivalent to the innermost electron shell of the Bohr model of the atom. It is called the 1s orbital because it is spherical around the nucleus. The 1s orbital is always filled before any other orbital.
- Mass number of atom = 23
- Atomic number of atom = 11
As, the number of electrons in an atom is equal to an atomic number of the element.
So, number of electrons = 11
Secondly , number of protons are equal to number of electrons.
So, number of protons = 11
And number of neutrons = mass number - atomic number = 23−11 = 12
So, number of neutrons = 12
Thus , the atom contains 11 protons, 11 electrons, 12 neutrons.
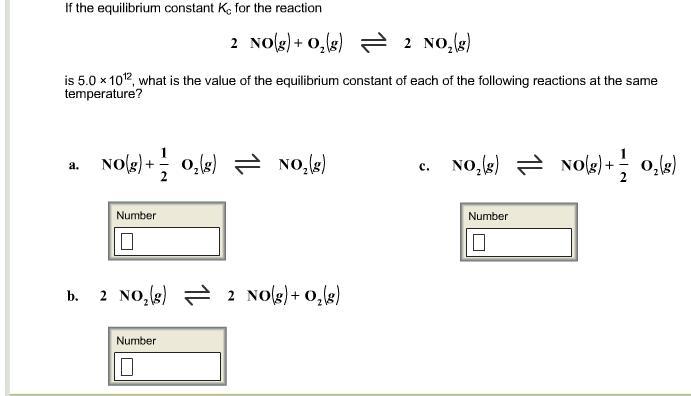
The equilibrium constant K
c
for the reaction below is 5.00×10
12
. 2NO(g)+O
2
(g)⇌2NO
2
(g) What is the value of the equilibrium constant of each of the following reactions at the same temperature? 1st attempt Part 1 NO(g)+
2
1
O
2
( g)⇌NO
2
( g) 2NO
2
(g)⇌2NO(g)+O
2
(g) Part 3 NO
2
(g)⇌NO(g)+
2
1
O
2
(g)
Answers
From the question, the equilibrium constant in each case is;
\(1) 2.2 * 10^6\\2)2 * 10^-13\\3) 4.5 * 10^-7\)
What is the equilibrium constant?The equilibrium constant is a dimensionless value and is independent of the initial concentrations of the reactants and products. It represents the ratio of the forward reaction rate to the reverse reaction rate at equilibrium.
We know that we can be able to obtain the equilibrium constant of the reaction in question by manipulating the equilibrium constant of the given equation.
In the first case;
\(K = (5 * 10^12)^1/2\\= 2.2 * 10^6\)
Second case;
\(K = (5 * 10^12)^-1\\K = 2 * 10^-13\)
Third case;
\(K = (5 * 10^12)^-1/2\\K = 4.5 * 10^-7\)
Learn more about equilibrium constant:https://brainly.com/question/28559466
#SPJ4
how many iron atoms are there in 5.33 mol of iron(iii) chloride
Answers
Answer:
The formula shows that there is one atoms of iron in each formula unit of FeCl3, and by definition the number of molecules or formula units in a mole is Avogadro's Number. Therefore, 5.33 moles contains 5.33 X Avogadro's Number of atoms, which is 3.21 X 1024 atoms, to the justified number of significant digits.
Answer:
The formula shows that there is one atoms of iron in each formula unit of FeCl3 , and by definition the number of molecules or formula units in a mole is Avogadro's Number . Therefore , 5.33 moles contains 5.33 X Avogadro's Number of atoms , which is 3.21 X 1024 atoms , to the justified number of significant digits .
how would you prepare 10.0 ml of a 0.25% m/v hcl solution if 1% m/v hcl was available? how much 1% m/v hcl is needed? how much distilled water is used?
Answers
To prepare 10.0 ml of a 0.25% m/v HCl solution, you will need to mix 2.5 ml of 1% m/v HCl solution with 7.5 ml of distilled water.
To make 10.0 ml of a 0.25% m/v HCl solution from 1% m/v HCl, dilute the 1% m/v HCl solution by a factor of 4.
Here's how to figure out how much 1% m/v HCl and distilled water you'll need:
Determine the concentration of HCl in the final solution:
A 0.25% m/v HCl solution contains 0.25 grams of HCl per 100 mL.
As a result, there will be 0.25 g/100 ml x 10 ml = 0.025 g of HCl in 10 ml of solution.
Determine the quantity of 1% m/v HCl required:
1% m/v HCl implies that there is one gram of HCl in every 100 ml of solution. You will need the following to make 0.025 g of HCl:
0.025 g/1 g x 100 ml = 2.5 ml of 1% m/v HCl solution
Determine the amount of distilled water required:
To prepare the remaining 7.5 ml of solution, use the following :
10 ml - 2.5 ml = 7.5 ml of distilled water
For more such questions on solution, click on:
https://brainly.com/question/23269908
#SPJ4
PLS HELP
Carbon (C) has an isotope that has an atomic number of 6 and an atomic mass of 13. Answer the following questions about this isotope.
How would it be identified in isotope notation?
How many neutrons does this isotope have?
Answers
Answer:
\( \frac{13}{6} \: c\)
Explanation:
The total isotopic mass is 13 (7 neutrons, 6 protons because this is Carbon)
50 POINTS
a 6.7g piece of rock boiled to 100.0 degrees celsius is placed in 100.0 mL of water with an initial temperature of 23 degrees celsius. the equilibrium temperature when the rock is added is 45 degrees celsius. what is the specific heat of the rock?
Answers
q = m * c * ΔT
where q is the heat absorbed or released, m is the mass of the substance, c is the specific heat of the substance, and ΔT is the change in temperature.
In this case, the heat released by the rock is equal to the heat absorbed by the water, so we can write:
q_rock = -q_water
where q_rock is the heat released by the rock and q_water is the heat absorbed by the water.
The heat released by the rock can be calculated as:
q_rock = m_rock * c_rock * ΔT
where m_rock is the mass of the rock and c_rock is the specific heat of the rock. We know that the mass of the rock is 6.7 g and the ΔT is 45 - 100 = -55 degrees Celsius (because the rock is losing heat to the water).
The heat absorbed by the water can be calculated as:
q_water = m_water * c_water * ΔT
where m_water is the mass of the water and c_water is the specific heat of water. We know that the mass of the water is 100.0 g (which is equivalent to 100.0 mL) and the ΔT is 45 - 23 = 22 degrees Celsius (because the water is gaining heat from the rock).
Since q_rock = -q_water, we can set the two equations equal to each other and solve for c_rock:
m_rock * c_rock * ΔT = -m_water * c_water * ΔT
c_rock = -m_water * c_water * ΔT / (m_rock * ΔT)
Plugging in the values, we get:
c_rock = -(100.0 g) * (4.184 J/g°C) * (22°C) / [(6.7 g) * (-55°C)]
c_rock = 0.811 J/g°C
Therefore, the specific heat of the rock is 0.811 J/g°C.
Answer:
To calculate the specific heat of the rock, you can use the formula for heat transfer: Q = mcΔT, where Q is the heat transferred, m is the mass of the substance, c is the specific heat capacity and ΔT is the change in temperature.
In this case, we can assume that the heat lost by the rock is equal to the heat gained by the water. Therefore:
Q(rock) = Q(water)
m(rock)c(rock)(T(final) - T(initial, rock)) = m(water)c(water)(T(final) - T(initial, water))
where m(rock) = 6.7 g, T(initial, rock) = 100.0°C, T(final) = 45°C, m(water) = 100.0 g (assuming the density of water is 1 g/mL), c(water) = 4.18 J/g°C (specific heat capacity of water), and T(initial, water) = 23°C.
Substituting these values into the equation above and solving for c(rock), we get:
c(rock) = (m(water)c(water)(T(final) - T(initial, water))) / (m(rock)(T(final) - T(initial, rock)))
c(rock) = (100.0 g * 4.18 J/g°C * (45°C - 23°C)) / (6.7 g * (45°C - 100.0°C))
c(rock) ≈ 1.26 J/g°C
So the specific heat of the rock is approximately 1.26 J/g°C.
One of the simplest ways to increase the dissolving rate of a spoonful of sugar in a cup of water is to:
a. chill the water.
b. stir the water.
c. evaporate the water.
d. use a sugar cube instead of granulated sugar.
Answers
Answer:
b. Stir the water
Explanation:
HELP ME PLEASEEEEEEE

Answers
Answer:
c,b not sure,c,v,b hope it will help u
if the sample of chips used to make the filtrate weighed 75.5 kg , how much nacl nacl is present in one serving (155 gg ) of chips?
Answers
The amount of table salt in one serving or 155g of chips is 0.608g if the sample of chips used to make the filtrate weighed 75.5 kg.
By undergoing titration by Mohr's method with AgNO₃ and KCl, The chemical reaction is given by,
AgNO3(aq) + NaCl(aq) --> AgCl(s) + NaNO3(aq)
No moles of KCI are given by W/G.M.Wt
= 0.5/74.55
= 0.006707 moles of KCI
Then, from the reaction,
no of moles of KCI = no of moles of AgNO₃
Therefore,
molarity of AgNO₃ = no of moles/volume in L
= 0.006707/0.0625
= 0.107312M
47.2 ml of AgNO₃ solution to precipitate all of the chlorides.
Then, no of moles of Cl⁻ = molarity x volume in L
On substituting,
= 0.107312*0.0472
= 0.005065 moles
no of moles of Cl⁻ = no of moles of NaCl
mass of NaCl = no of moles of NaCl x gram molar mass of NaCl
= 0.005065 x 58.5
= 0.2963g of NaCl
155/75.5. = 2.05298013 chips
2.05298013x 0.2963 = 0.608298013 g
Therefore, 0.608g of NaCl is in 115g of chips.
To know more about food chemistry, click below:
https://brainly.com/question/29517724
#SPJ4
(1) What is the element with an electron configuration of 1s22s22p63s23p64s23d6?
(2) What is the element with an electron configuration of 1s22s22p63s23p6?
(3) What is the name of the element with a valence electron configuration of 4s1?
(4) What is the name of the element with a valence electron configuration of 2s22p5?
A. An element with the valence electron configuration 3s1 would form a monatomic ion with a charge of .
In order to form this ion, the element will (lose or gain) (1/2/3/4/5/6/7/8) electron(s) from/into the ( s/p/d/f/ s + p /s + d/ p + d) subshell(s).
B. An element with the valence electron configuration 3s23p3 would form a monatomic ion with a charge of .
In order to form this ion, the element will (lose or gain) (1/2/3/4/5/6/7/8) electron(s) from/into the( s/p/d/f/ s + p /s + d/ p + d) subshell(s).
Answers
(1) The element with an electron configuration of 1s22s22p63s23p64s23d6 is Iron (Fe).
The above electronic configuration is the repesentation of the electrons presents in different orbitals of the iron(Fe) .we have seen that all orbitals is completely filled before D orbital.
(2) The element with an electron configuration of 1s22s22p63s23p6 is Argon (Ar)
The above electronic configuration is the repesentation of the electrons presents in different orbitals of the Argon(Ar) .we have seen that all orbitals is completely filled.
(3) The name of the element with a valence electron configuration of 4s1 is Potassium (K)
The above electronic configuration is the repesentation of the electrons presents in different orbitals of thePotassium (K) .we have seen that all orbitals is completely filled before 4s1.
(4) The name of the element with a valence electron configuration of 2s22p5 is Flourine (F)
The above electronic configuration is the repesentation of the electrons presents in different orbitals of the Flourine (F) .we have seen that all orbitals is completely filled before 2p5.
A. An element with the valence electron configuration 3s1 would form a monatomic ion with a charge of +1. In order to form this ion, the element will lose one electron from the 3s subshell.
B. An element with the valence electron configuration 3s23p3 would form a triatomic ion with a charge of -3. In order to form this ion, the element will gain three electrons from the 3s and 3p subshells.
Elements achieve an inert gas configuration by gaining or losing electrons to attain a full valence shell of electrons, resulting in a stable electron configuration. This is also known as having a complete octet in their outermost energy level.
To know more about valence electron click below:
https://brainly.com/question/28977387#
#SPJ4
Question 2 of 11 What will happen to the particles of a substance that is cooled to OK? A. They will stop moving. B. They will speed up. C. They will form a liquid. D. They will gain thermal energy.
plsssss help fast plss
Answers
one of the benefits of utilizing an automated chest compression device is that:
Answers
One of the benefits of utilizing an automated chest compression device is that it provides consistent and effective compressions, which can lead to better outcomes for patients in cardiac arrest. These devices are designed to deliver compressions at a consistent rate and depth, which is difficult to achieve consistently with manual compressions.
Manual chest compressions are physically demanding and can result in fatigue, which can impact the quality of compressions over time.
Automated devices, on the other hand, are designed to provide consistent compressions for extended periods without fatigue, which can improve outcomes for patients in cardiac arrest.
Another benefit of automated chest compression devices is that they can free up medical personnel to perform other tasks during a cardiac arrest.
When performing manual compressions, medical personnel must stay in close proximity to the patient and continue to provide compressions for extended periods.
With an automated device, medical personnel can step back and focus on other tasks, such as administering medications or managing the airway, while the device provides consistent compressions.
In addition, automated devices can be used in a variety of settings, including hospitals, ambulances, and other pre-hospital settings.
This allows for consistent care across different locations and can help improve outcomes for patients who experience cardiac arrest.
Overall, the use of automated chest compression devices can provide significant benefits for patients in cardiac arrest, including more consistent and effective compressions, increased efficiency, and improved outcomes.
For more such questions on cardiac arrest
https://brainly.com/question/30215118
#SPJ8
what type of planing hull handles rough water the best?
Answers
The best type of planning hull for handling rough water is a deep-V hull. A deep-V hull that has a sharp, angled V-shape at the bottom and is designed to cut through the water.
This design allows the hull to ride up over waves and maintain stability and control in choppy seas. The deep-V also helps to reduce spray, making it more comfortable for passengers. Additionally, the deep-V hull has greater speed and maneuverability than other types of planing hulls, allowing it to maneuver effectively in rough water. The deep-vee hull also helps the boat to cut through waves and maintain its course, while the sharp bow and stern reduce drag and increase the boat’s speed.
To learn more about water click here https://brainly.com/question/28465561
#SPJ4
What effect would increasing the amount of lauric acid present in the test tube have on the freezing point and the shape/ features of the graph?
(This is a freezing point of lauric acid lab question require at least five sentences)
Answers
Lauric acid, 300 g
Benzoic acid, 30 g
Paper towels
Advance Preparation
It is important that you do the laboratory yourself beforehand. Time may be saved by pre-filling the test-tubes with about 25 g of lauric acid and having the hot and warm water baths ready. [NOTE: The mass of the lauric acid in the test-tubes must be determined to the nearest gram.]
How much heat must be transferred to 55 g of ice to change the ice's
temperature from -13°C to -5.0°C? (The specific heat capacity of ice is 2.11
J/g.°C)
Answers
Q= m x c x t
M= 55 g
C=2.11
T=8
SCOPE OF NUCLEAR ENERGY IN NEPAL"
Answers
Answer:
intruducing nuclear in a country like nepal where the knowledge of nuclear science is limited is a major task.
Explanation:
(maybe it will help if not sorry because my answer is wrong)
Nepal is a landlocked country that has been facing challenges in meeting its energy demand. The country is heavily dependent on imported fossil fuels, which are expensive and have negative environmental impacts.
Nuclear energy is the energy that is released from the nucleus of an atom through a process called nuclear reaction. Nuclear reactions can occur spontaneously or can be induced by bombarding the nucleus with particles such as neutrons.
However, Nepal currently does not have any nuclear power plants, and the country has not developed any nuclear energy infrastructure. Therefore, the development of nuclear energy in Nepal would require significant investment in terms of time, money, and human resources. Additionally, there are concerns about the safety and security of nuclear energy, and these issues would need to be addressed before nuclear energy could be developed in Nepal.
Despite these challenges, there are some potential benefits to the development of nuclear energy in Nepal. Nuclear energy has the potential to provide a reliable source of energy that is not dependent on imported fossil fuels. Additionally, nuclear energy is a low-carbon source of energy that could help Nepal reduce its greenhouse gas emissions and mitigate the impacts of climate change.
In conclusion, the development of nuclear energy in Nepal is a complex issue that requires careful consideration of the potential benefits and risks. While nuclear energy could provide a reliable and low-carbon source of energy, there are significant challenges that would need to be addressed before nuclear energy could be developed in Nepal.
Learn more about nuclear energy here:
https://brainly.com/question/2409175
#SPJ4
balancing chemical equation helpp me H3PO4+KOH---> K3PO4+H2O some one balance this please help me
Answers
Answer:
H3PO4 + 3KOH -> K3PO4 + 3H20
Answer:
hope its helpful to uh....

. Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
(a) 0.200 M HCl
(b) 0.0143 M NaOH
(c) 3.0 M HNO3
(d) 0.0031 M Ca(OH)2
Answers
pH= -log [H3O+]
PH=-log (0.200)
= 0.699
poH= 14-0.699
= 13.301
b. NaOH:
PoH= -log [OH-]
= -log (0.0143)
= 1.845
pH= 14-poH
= 14- 1.845
= 12.16
c. HNO3:
PH= -log[H3O+]
=-log(3.0)
= -0.4771
poH= 14-pH
= 14-9-0.4771
= 14.4771
pH= -0.4771, poH= 14.4771
d. [Ca(OH)2] = 0.0031M
[OH-]= 2X0.0031
[OH-] = 0.0062M
PoH= - log[OH-]
=-log(0.0062)
=-log(6.2x10-3)
=-(-2.21)
= 2.21
PH=14-poH
=14-2.21
=11.79
POH=2.21, PH= 11.79
Based on the molarity of the solutions;
For 0.200 M HCl; pH = 0.699, pOH = 13.301For 0.0143 M NaOH; pOH = 1.845, pH = 12.16For 3.0 M HNO3; pH = -0.4771, poH = 14.4771For 0.0031 M Ca(OH)2; pOH = 2.21, pH = 11.79What pHand pOH?pH is the negative logarithm to base ten of the hydrogen ions concentration of a solution.
pH = -log[H+]pOH is the negative logarithm to base ten of the hydroxide ions concentration.
pOH = -log[OH-]Also;
pH + pOH = 14For HCl:
pH = -log [H3O+]
pH =-log (0.200)
pH = 0.699
Then;
poH= 14-0.699
pOH = 13.301
For NaOH:
pOH= -log [OH-]
= -log (0.0143)
pOH = 1.845
Then;
pH= 14-poH
= 14- 1.845
pH = 12.16
For HNO3:
pH= -log[H3O+]
=-log(3.0)
= -0.4771
Then;
pOH = 14-9-0.4771
pOH = 14.4771
For [Ca(OH)2]
molarity = 0.0031M
2 moles of OH- are produced
[OH-]= 2 × 0.0031
[OH-] = 0.0062M
pOH = - log[OH-]
=-log(0.0062)
=-log(6.2x10-3)
=-(-2.21)
pOH = 2.21
Then;
pH =14-2.21
pH =11.79
Learn more about pH and pOH at: https://brainly.com/question/13557815
extra step here! can you catch it while dropping off your recycling you are over come by the urge to weigh the tin cans you brought in. you find that the mass of cans in the box you brought had a mass of 23kg. how many moles do you have
Answers
55.845 g/mol of the mass of cans in the box we brought had a mass of 23kg. 411.74 mol moles we have.
To determine the number of moles of tin cans you have, we need to know the molar mass of the metal that the cans are made of. Tin cans are usually made of steel, which is an alloy made primarily of iron and carbon, with small amounts of other elements.
To convert the mass of the cans (23 kg) to moles, we need to use the following formula:
moles = mass / molar mass
moles = 23,000 g / 55.845 g/mol = 411.74 mol
Therefore, the number of moles of iron in the 23 kg of tin cans is approximately 411.74 mol.
Learn more about molar mass here:
https://brainly.com/question/12127540
#SPJ4
HELP PLSSS ILL GIVE BRAINLIEST TO WHOEVER HELPS plsss

Answers
Answer:
they all look like they are
Explanation:
Answer:
Only A and B are redox
Explanation:
Redox reactions are ones where elements change their oxidation states. That means any reactions that involve free elements that are also in compounds, ex: Na in a) is in both 2Na and 2NaCl are redox because their oxidation states change from 0 to whatever in the compound. This means A and B are redox reactions.
For C, (CO3) has a charge of -2, and reactants have charges C = +4, O = -2, and H = +1
The product compounds are all zero charge, so charges C = +4 and O = -2 makes CO2 charge 0 and H = +1 and O = -2 also gives H2O a zero charge. Since al elements of reactants are products do not have a change in charge, it is not redox.
Note that Oxygen is almost always at a charge of -2, that should help you with calculations.
Similarly in d), (NO3) has charge -1, with charge N = +5, O = -2, and Pb = +2.
K tends to have charge +1 and I -1.
For d) reactants, Pb = +2 and I = -1 still gives PbI2 a zero charge. KNO3 also leaves it a zero charge.
Therefore d) also does not have any charge change in elements so it is not redox
what are the features of a standard hydrogen electrode? a temperature of 298 k a carbon electrode hydrogen gas at 1.01 x 10^5 pa (1 atm) pressure
Answers
The features of a standard hydrogen electrode are :
1. Temperature of 298 K (25°C)
2. Carbon electrode
3. Hydrogen gas at 1.01 x 10^5 Pa (1 atm) pressure
4. Electrolyte solution containing a hydrogen ion activity of 1 mol/L
5. Platinum wire as the current collector
6. A Potential of 0.00 V (relative to the hydrogen gas)
These features are what make up the Standard Hydrogen Electrode (SHE). The temperature of 298 K is the temperature at which the SHE is calibrated and is the standard temperature used in most laboratory experiments. The carbon electrode serves as the interface between the hydrogen gas and the electrolyte, and the hydrogen gas is held at 1.01 x 10^5 Pa (1 atm) pressure. The electrolyte solution contains a hydrogen ion activity of 1 mol/L, which is necessary for the electrode to function properly. A platinum wire is used as the current collector, and the electrode has a potential of 0.00 V, relative to the hydrogen gas. All of these features are necessary for the SHE to function properly and for the electrode to serve as the reference for all other electrochemical measurements.
To know more about electrodes refer to the link brainly.com/question/17060277
#SPJ4
identify the incorrect statement(s). a solution _____ i. can be a solid, liquid, or gas. ii. can be heterogeneous or homogeneous. iii. is a homogeneous mixture.
Answers
The incorrect statement in this case is statement i. A solution cannot be a gas. A gas is not a solution on its own, but it can be a component in a mixture or a solution.
A mixture is a combination of two or more substances that are not chemically combined and can exist in any state - solid, liquid, or gas. A solution, on the other hand, is a homogeneous mixture where one substance (the solute) is dissolved in another substance (the solvent). The solute can be a solid, liquid, or gas, but the solvent must be a liquid.
Statement ii is correct. A solution can be homogeneous or heterogeneous. A homogeneous solution has uniform composition throughout, meaning that the solute is evenly distributed in the solvent. In contrast, a heterogeneous solution has non-uniform composition, meaning that the solute is not evenly distributed in the solvent.
Statement iii is also correct. A solution is a homogeneous mixture. This means that the solute is evenly distributed in the solvent to create a uniform composition. A homogeneous mixture has the same properties and composition throughout, and the components cannot be visibly distinguished from each other.
In summary, a solution cannot be a gas, but it can be a homogeneous mixture of a solid, liquid, or gas dissolved in a liquid solvent. A mixture can exist in any state and can be homogeneous or heterogeneous, while a solution is always a homogeneous mixture.
To learn more about gases:
https://brainly.com/question/22817140
#SPJ11
HELP ME THIS IS FOR TODAY!!!!!

Answers
Answer:
4. 0.5 kilometers (1st option)
5. 0.1 00 liters (last option)
Explanation:
500 meters to kilometers
4.
1 kilometer = 1000 meters
We use the formula SBD (SMALL TO BIG DIVIDE) i hope this helps you
500 ÷ 1000
= 0.5 kilometers
5.
100 milliliter to liter
SBD
100 ÷ 1000
= 0.1 00 liters
Which of the following happens to the particles when the temperature of liquid water drops?
kinetic energy decreases
kinetic energy increases and then decreases
kinetic energy increases
kinetic energy stays constant
Answers
Answer:
The Kinetic energy decreases
Explanation:
because the speed of the molecules slows down.
zinc (s)+ sulfuric acid (aq) → zinc sulfate (aq)+hydrogen (g)
Answers
Answer:
What exactly are you asking?
Explanation:
I am unsure, this reaction is a balanced reaction by default if that was what you were asking.
What is the name of the new technology whereby a glass fiber carries as much information as hundreds of copper wires?
Answers
Answer:
Fibre optic technology.
Explanation:
Fibre optic technology, as the name implies, uses light pulses to transmit data via strands of glass or plastic. It's the preferred technology for the government's National Broadband Network (NBN), which offers 100Mbps+ download speeds.
How many molecules of nh3 can be produced from the reaction of 74. 2 g of n2 and 14. 0 moles of h2?.
Answers
From the reaction of 74.2 g of N2 and 14.0 moles of H2, approximately 3.18 x 10^24 molecules of NH3 can be produced.
To determine how many molecules of NH3 can be produced from the reaction of 74.2 g of N2 and 14.0 moles of H2, we need to use the balanced chemical equation for the reaction:
N2 + 3H2 -> 2NH3
First, let's convert the mass of N2 to moles. The molar mass of N2 is 28.02 g/mol, so:
74.2 g N2 * (1 mol N2/28.02 g N2) = 2.64 mol N2
Next, we need to determine the limiting reactant. To do this, we compare the moles of N2 and H2 in a 1:3 ratio. Since there are 2.64 moles of N2 and 14.0 moles of H2, we divide the moles of each reactant by their respective coefficients in the balanced equation:
2.64 mol N2 / 1 = 2.64
14.0 mol H2 / 3 = 4.67
The mole ratio tells us that for every 1 mole of N2, we need 3 moles of H2. Since we have more moles of H2 (4.67) than moles of N2 (2.64), H2 is in excess, and N2 is the limiting reactant.
Now we can calculate the moles of NH3 produced. According to the balanced equation, 1 mole of N2 produces 2 moles of NH3. So:
2.64 mol N2 * (2 mol NH3/1 mol N2) = 5.28 mol NH3
Finally, we can convert moles of NH3 to molecules. Avogadro's number tells us that 1 mole of any substance contains 6.022 x 10^23 molecules. Therefore:
5.28 mol NH3 * (6.022 x 10^23 molecules NH3/1 mol NH3) = 3.18 x 10^24 molecules NH3
Therefore, from the reaction of 74.2 g of N2 and 14.0 moles of H2, approximately 3.18 x 10^24 molecules of NH3 can be produced.
Learn more about reaction here:-
https://brainly.com/question/16737295
#SPJ11
You had a closed tank of air at a pressure of 4 atm and temperature of 20 degrees Celsius. When the tank and the air are heated to 40 degrees Celsius, what is the pressure if the volume remains constant?
Answers
Answer:
The pressure will be 4.27 atm.
Explanation:
Gay-Lussac's law can be expressed mathematically as follows:
\(\frac{P}{T} =k\)
Where P = pressure, T = temperature, K = Constant
This law indicates that the quotient between pressure and temperature is constant.
This law indicates that, as long as the volume of the container containing the gas is constant, as the temperature increases, the gas molecules move faster. Then the number of collisions with the walls increases, that is, the pressure increases. That is, the pressure of the gas is directly proportional to its temperature.
In short, when there is a constant volume, as the temperature increases, the pressure of the gas increases. And when the temperature is decreased, the pressure of the gas decreases.
You want to study two different states, an initial state and a final state. You have a gas that is at a pressure P1 and a temperature T1 at the beginning of the experiment. By varying the temperature to a new value T2, then the pressure will change to P2, and the following will be fulfilled:
\(\frac{P1}{T1} =\frac{P2}{T2}\)
In this case:
P1= 4 atmT1= 20 C= 293 K (being 0 C= 273 K)P2= ?T2= 40 C= 313 KReplacing:
\(\frac{4 atm}{293 K} =\frac{P2}{313 K}\)
Solving:
\(P2= 313 K* \frac{4 atm}{293 K}\)
P2= 4.27 atm
The pressure will be 4.27 atm.
Determine the molecular formula of the compound with an empirical formula of CH and a molar mass of 65.09 g/mol.
Answers
We have to determine the molecular formula of a compound. We know that its empirical formula is CH. To find the molecular formula we have to compare the molar mass of the empirical formula with the molar mass of the compound (65.09 g/mol).
Let's start determining the molar mass of the empirical formula. The atomic masses of C and H are:
C: 12.01 amu H: 1.01 amu
With those values we can calculate the molar mass of CH:
molar mass of CH = 1 * 12.01 + 1 * 1.01
molar mass of CH = 13.02 g/mol
If we divide the molar mass by the molar mass of the empirical formula we will get the relationship between them.
x = molar mass/molar mass of the empirical formula
x = 65.09 g/mol / (13.02 g/mol)
x = 5
Finally to get the molecular formula we have to multiply each element by that ratio.
Answer: C₅H₅ is the molecular formula.