How many molecules of n2o4 are in 76. 3 g n2o4? the molar mass of n2o4 is 92. 02 g/mol.
Answers
The number of molecules of n2o4 in 76.3 g n2o4 is 4.986 x 1023 molecules.
To determine the number of molecules of n2o4 in 76.3g n2o4, it is important to follow some steps that will ensure the accuracy of the answer.
Steps: First, compute the number of moles of n2o4 from the mass, using the formula; Number of moles = Mass of the substance/Molar mass number of moles of n2o4 = 76.3 g/92.02 g/mol = 0.829 moles.
Next, use Avogadro's number (6.022 x 1023) to convert the number of moles to the number of molecules. It states that one mole of a substance contains 6.022 x 1023 molecules.
The number of molecules of n2o4 = a number of moles of n2o4 x Avogadro's number
Number of molecules of n2o4 = 0.829 mol x 6.022 x 1023 molecules/mole
= 4.986 x 1023 molecules.
Finally, conclude by stating that there are 4.986 x 1023 molecules of n2o4 in 76.3g n2o4.In summary, the number of molecules of n2o4 in 76.3 g n2o4 is 4.986 x 1023 molecules.
Learn more about Avogadro's number in:
brainly.com/question/28812626
#SPJ11
Related Questions
When the temperature and number of particles of a gas are constant, which of the following is also constant?

Answers
Answer:
i dont see the pic
Explanation:
Answer:
According to Gas Law
Volume is inversely proportional to pressure, if the number of particles and the temperature are constant. There are two ways for the pressure to remain the same as the volume increases.
1. Determine the molecular formula of an oxide of iron in which the mass of iron and oxygen are 69.9% and 30% respectively given that the molar mass of the oxide 159.898/mol, find the empirical and molecular formula.
2. a crystalline salt when heated becomes anhydrous and loses 51.2% of its weight the anhydrous salt analysis gave the percent composition as magnesium is equal to 20.0% and sulphur is equal to 26.66% and oxygen is equal to 53.33%.
3. In three moles of Ethane calculate the following
1. calculate number of carbon atoms.
2. number of moles of hydrogen atoms
3. number of molecules of Ethane.
Answers
1a. The empirical formula of the compound is Fe₂O₃
1b. The molecular formula of the compound is Fe₂O₃
2a. The molecular formula of the anhydrous salt is MgSO₄
2b. The formula of the crystalline salt is MgSO₄.7H₂O
3i. The number of mole of carbon atoms in the compound is 6 moles
3ii. The number of mole of hydrogen atoms in the compound is 18 moles
3iii. The number of molecules in 3 moles of ethane is 1.806×10²⁴ molecules
1a. How to determine the empirical formulaFe = 69.9%O = 30%Empirical formula =?Divide by their molar mass
Fe = 69.9 / 56 = 1.248
O = 30 / 16 = 1.875
Divide by the smallest
Fe = 1.248 / 1.248 = 1
O = 1.875 / 1.248 = 3/2
Multiply by 2 to express in whole number
Fe = 1 × 2 = 2
O = 3/2 × 2 = 3
Thus, the empirical formula of the compound is Fe₂O₃
1b. How to determine the molecular formulaEmpirical formula = Fe₂O₃Molar mass of compound = 159.89 g/molMolecular formula = ?Molecular formula = empirical × n = molar mass
[Fe₂O₃]n = 159.89
[(56×2) + (16×3)]n = 159.89
160n = 159.89
n = 159.89 / 160
n = 1
Molecular formula = [Fe₂O₃]n
Molecular formula = [Fe₂O₃] × 1
Molecular formula = Fe₂O₃
2a. How to determine the molecual formula of the anhydrous saltWe'll begin by calculating the empirical formula
Mg = 20.0% S = 26.66% O = 53.33%Empirical formula =?Divide by their molar mass
Mg = 20.0 / 24 = 0.83
S = 26.66 / 32 = 0.83
O = 53.33 / 16 = 3.33
Divide by the smallest
Mg = 0.83 / 0.83 = 1
S = 0.83 / 0.83 = 1
O = 3.33 / 0.83 = 4
Thus, the empirical formula of the anhydrous salt is MgSO₄
The molecular formula of the anhydrous salt can be obtained as follow:
Empirical formula = MgSO₄Molar mass of compound = 120 g/molMolecular formula = ?Molecular formula = empirical × n = molar mass
[MgSO₄]n = 120
[24 + 32 + (16×4)]n = 159.89
120n = 120
n = 120 / 120
n = 1
Molecular formula = [MgSO₄]n
Molecular formula = [MgSO₄] × 1
Molecular formula = MgSO₄
2b. How to determine the formula of the crystalline saltWater (H₂O) = 51.2%Anhydrous salt (MgSO₄) = 100 - 51.2 = 48.8%Formula of crystalline salt =?Divide by their molar mass
MgSO₄ = 48.8 / 120 = 0.4
H₂O = 51.2 / 18 = 2.8
Divide by the smallest
MgSO₄ = 0.4 / 0.4 = 1
H₂O = 2.8 / 0.4 = 7
Thus, the formula of the crystalline salt is MgSO₄.7H₂O
3i. How to determine the mole of carbon atoms in 3 moles of C₂H₆1 mole of C₂H₆ contains 2 moles of carbon atoms.
Therefore,
3 moles of C₂H₆ will contain = 3 × 2 = 6 moles of carbon atoms
3ii. How to determine the mole of hydrogen atoms in 3 moles of C₂H₆1 mole of C₂H₆ contains 6 moles of hydrogen atoms.
Therefore,
3 moles of C₂H₆ will contain = 3 × 6 = 18 moles of hydrogen atoms
3iii. How to determine the number of moleculesFrom Avogadro's hypothesis,
1 mole of ethane = 6.02×10²³ molecules
Therefore,
3 moles of ethane = 3 × 6.02×10²³ molecules
3 moles of ethane = 1.806×10²⁴ molecules
Learn more about empirical formula:
https://brainly.com/question/24297883
Learn more about Avogadro's number:
https://brainly.com/question/26141731
#SPJ1
Complete question
2. A crystalline salt when heated becomes anhydrous and loses 51.2% of its weight the anhydrous salt analysis gave the percent composition as magnesium is equal to 20.0% and sulphur is equal to 26.66% and oxygen is equal to 53.33%. Ccalculate the molecular formula of the anhydrous and the crystalline salt. The molecular weight of the anhydrous salt is 120
a solution contains 25.0 g ethanol (c2h5oh; molar mass 46.07 g/mol) in 500. g h2o (molar mass 18.02 g/mol) at 23oc. if the vapor pressure of pure h2o at this temperature is 20.57 torr, what is the vapor pressure of the solution?
Answers
The partial pressure of the solution is 0.39 torr.
What is Raoult's Law?The Raoult's Law states that, the partial pressure of the solution is equal to the product of the mole fraction of the solute and the partial pressure of the pure solvent.
Hence;
Partial pressure of the pure solvent = 20.57 torr
Moles of water = 500 g/18 g/mol = 27.8 moles
Moles of ethanol = 25.0 g/46 g/mol = 0.54 moles
Total number of moles = 27.8 moles + 0.54 moles = 28.34 moles
Hence;
Partial pressure of solution = 0.54 moles/28.34 moles * 20.57 torr
= 0.39 torr
Learn more about Raoult's Law: https://brainly.com/question/28304759
#SPJ4
Which of the following is NOT a way to prevent invasive species from becoming a bigger issue? A Stay updated on research around new invasive species in your community. B Introduce unknown species to new environments for research purposes. Educate ourselves and others on guidelines around invasive species. Read up on local environmental laws before traveling with any animals or plants.
Answers
Answer:
B
Explanation:
B
In an experiment (first order system), the water in a beaker is heated from temperature of 20
∘
C to the boiling point of 100
∘
C. The time taken for the temperature to reach 100
∘
C is 120 seconds. Derive the transfer function of the boiling process.
Answers
The exponential function is always positive, we can conclude that there is no solution to this equation. This implies that the given data is not consistent with a first-order system.
The transfer function of a system describes the relationship between the input and output signals of the system in the frequency domain. However, the boiling process itself does not have a standard transfer function because it is a complex and dynamic phenomenon influenced by various factors such as temperature, pressure, fluid properties, and heat transfer mechanisms.
To derive the transfer function of the boiling process, we need to understand the dynamics of the system. In this case, we have a first-order system where the water in a beaker is heated from a temperature of 20 °C to the boiling point of 100 °C. The time taken for the temperature to reach 100 °C is given as 120 seconds.
To begin, let's define the input and output variables of the system. The input variable is the heating power or energy applied to the beaker, and the output variable is the temperature of the water.
The transfer function is a mathematical representation of the relationship between the input and output of a system. In this case, the transfer function describes how the temperature of the water changes in response to the heating power.
Let's assume the transfer function is represented as G(s), where s is the complex frequency variable.
To derive the transfer function, we can use the time-domain response data provided. The first-order system response to a step input can be described by the following equation:
y(t) = K(1 - e^(-t/τ))
where y(t) is the output (temperature of the water), K is the steady-state gain, t is time, and τ is the time constant.
Given that the temperature reaches 100 °C after 120 seconds, we can substitute the values into the equation:
100 = K(1 - e^(-120/τ))
Simplifying the equation, we have:
1 - e^(-120/τ) = 100/K
Now, let's consider the initial condition where the water temperature is 20 °C at t = 0. Plugging these values into the equation, we have:
20 = K(1 - e^(-0/τ))
20 = K
Substituting this value of K into the previous equation, we get:
1 - e^(-120/τ) = 100/20
1 - e^(-120/τ) = 5
Now, let's solve for τ. Rearranging the equation, we have:
e^(-120/τ) = 1 - 5
e^(-120/τ) = -4
In summary, based on the provided information, it is not possible to derive the transfer function of the boiling process as a first-order system. Further information or clarification is needed to accurately determine the transfer function.
To know more about first-order system, visit:
https://brainly.com/question/33303904
#SPJ11
How many grams are equal to 413 kg in scientific notation
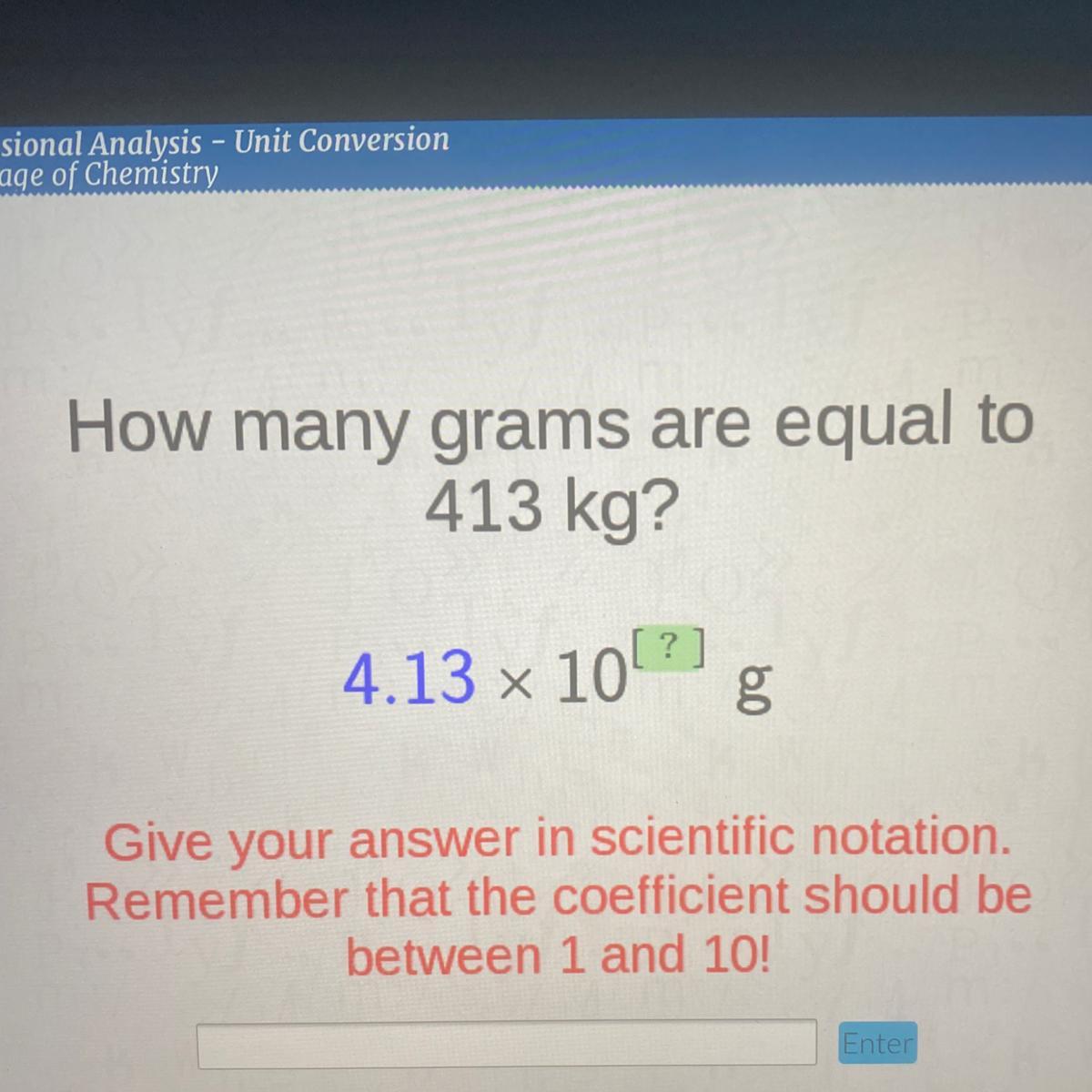
Answers
Answer:
\(4.13\) x \(10^{2}\) = 413 kg
Explanation:
4.13 x 10 = 41.3 then we multiply 41.3 by 10 agian = 41.3 x 10 = 413
so, we multiplied ten two times that = \(10^{2}\)
= 4.13 x \(10^{2}\)= 413 kg
Given that 1 kilogram = 1000grams, the value of 413 kilograms in grams, using scientific notation is 4.13 × 10⁵g.
How many grams are equal to 413 kg in scientific notation?Note that;
1 kilogram = 1000grams
Given that;
Mass in kilograms = 413kgMass in grams = ?Since, 1 kilogram = 1000grams
413 kilograms = ( 413 × 1000 )grams
413 kilograms = ( 413000 )grams
413 kilograms = 4.13 × 10⁵ g
Given that 1 kilogram = 1000grams, the value of 413 kilograms in grams, using scientific notation is 4.13 × 10⁵g.
Learn more about conversion of mass here: https://brainly.com/question/14147088
#SPJ9
Write a 600-word report discussing nuclear reactors. The report should include a description of the way a reactor works and the theory behind nuclear reactions. Be sure to answer these questions: What are breeder reactors? How are they different from regular nuclear reactors? What are their advantages and disadvantages?
Write your report in the essay box below.
PLEASESSSSS THE DUE DATE IS TOMORROW
Answers
Nuclear reactors are the main components of the nuclear power plants which are used for the production of heat through the process called fission.
What are breeder reactors?The nuclear reactors are divided into many types which include:
Pressurized water reactor,Boiling water reactor,Pressurized heavy water reactor, Light water graphite reactor and Breeder reactors.The Breeder reactors are the type of reactors that has the ability to yield more energy than it can consume which makes it economical.
The Breeder reactors are different from the regular nuclear reactors because it utilizes either uranium-238 or thorium, while regular nuclear reactors makes use of isotope uranium-235.
An advantage of Breeder reactors is that they produce more fuel that it consumes while it's disadvantage is that it's more dangerous than a regular reactor.
Learn more about nuclear reactors here:
https://brainly.com/question/14400638
#SPJ1
a metal (fw 340.1 g/mol) crystallizes into a body-centered cubic unit cell and has a radius of 2.87 angstrom. what is the density of this metal in g/cm3?
Answers
It is discovered that an unidentified metal crystallizes in a body-centered cubic lattice and has a density of 7.8748 g/cm3. The unit cell's edge measures 0.28664 nm.
If iron crystallizes in a body-centered cubic unit cell with an edge length of 287 pm, what is its density?A body-centered cubic unit cell with an edge length of 287 pm forms during the crystallization of iron metal. 287 p m . Iron has a molar mass of 55.85 gmol (1.85 gmol) and a density of 7.86 gcm (3.86 g c m)
Consequently, the formula for obtaining 7.97 g of iodine is: = 789.4 g x 7.97 g / 761.4 of NI3 = 6291.518 / 761.4 of NI3 = 8.26 g of NI3 Hence Grams.
To know more about density visit1:-
https://brainly.com/question/15164682
#SPJ4
I need help ASAP!!!! Which of the following is an advantage of using nuclear power plants to
produce electricity?
A) Radioactive waste from nuclear reactors is now being used to power cars,
B) There are no hazardous byproducts from nuclear reactions,
C) Nuclear power does not produce greenhouse gasees
Answers
Answer:
The answer is C.
Explanation:
Answer:
C is right i yeah C is right
what are the 3 specific ways an object can accelerate
Answers
Answer:
increasing speed, decreasing speed, or a change in direction.
Explanation:
i hope this helps
which biogeochemical cycle(s) is/are impacted by the burning of fossil fuels?
Answers
The burning of fossil fuels primarily impacts the carbon cycle, specifically the release of carbon dioxide (CO2) into the atmosphere. This increased concentration of CO2 contributes to the greenhouse effect and climate change. Additionally, the burning of fossil fuels can indirectly affect other biogeochemical cycles, such as the nitrogen cycle, through the production of nitrogen oxides (NOx) and their subsequent impacts on ecosystems.
The burning of fossil fuels, such as coal, oil, and natural gas, releases large amounts of carbon dioxide (CO2) into the atmosphere. This release disrupts the balance of the carbon cycle, which is responsible for the exchange and cycling of carbon between the atmosphere, land, and oceans. Fossil fuels, formed from ancient plant and animal matter, contain carbon that has been stored underground for millions of years. When burned for energy, the carbon is rapidly released as CO2, adding to the atmospheric pool of carbon and increasing the concentration of greenhouse gases.
The increased concentration of CO2 in the atmosphere contributes to the greenhouse effect, trapping heat and leading to global warming and climate change. This impacts various aspects of the Earth's systems, including temperature patterns, weather events, and sea-level rise.
Furthermore, the burning of fossil fuels can indirectly impact other biogeochemical cycles, such as the nitrogen cycle. Fossil fuel combustion releases nitrogen oxides (NOx), which are air pollutants that contribute to air pollution and acid rain. Nitrogen oxides can react with other atmospheric compounds and form nitric acid, which can be deposited onto land and water bodies through precipitation. This influx of nitrogen compounds can disrupt natural nitrogen cycling processes, affecting ecosystems, nutrient availability, and aquatic habitats.
In conclusion, the burning of fossil fuels primarily affects the carbon cycle by releasing carbon dioxide and contributing to climate change. It also has indirect impacts on other biogeochemical cycles, such as the nitrogen cycle, through the release of nitrogen oxides and subsequent ecological consequences. It is essential to reduce reliance on fossil fuels and transition to sustainable and renewable energy sources to mitigate these negative effects on biogeochemical cycles and promote a healthier planet.
To learn more about burning of fossil fuels : brainly.com/question/2029072
#SPJ11
What is the formula for Hydroxide
Answers
Answer:
OH-
Explanation:
it's consists of an oxygen and hydrogen atom held together by a covalent bond and carries a negative electric charge
why does hydrogen peroxide decomposes into water and oxygen?
Answers
Hydrogen peroxide decomposes into water and oxygen because it is a relatively unstable compound, and the decomposition reaction is exothermic, meaning that it releases energy.
Hydrogen peroxide is a compound made up of hydrogen and oxygen atoms, with the chemical formula H₂O₂. It is a relatively unstable compound and can break down into water and oxygen spontaneously. This decomposition reaction can be accelerated by the presence of catalysts such as heat, light, or certain metals.
The decomposition reaction of hydrogen peroxide is exothermic, meaning that it releases energy in the form of heat and light. This energy release can be observed as bubbles of oxygen gas form and escape from the liquid, while the remaining liquid turns into water.
The chemical reaction for the decomposition of hydrogen peroxide is as follows,
2H₂O₂ (hydrogen peroxide) → 2H₂O (water) + O₂ (oxygen)
To know more about the hydrogen peroxide, here
brainly.com/question/29102186
#SPJ4
Au HSO3 nomenclatura
Answers
differentiate between
Halogens and insert gases
Answers
Answer:
An inert gas is one that does not undergo chemical reactions
Noble gases refers to the right most group of the periodic table composed of helium, neon, argon, krypton, xenon, and radon. As you might have seen as an example in class, some noble gases can form chemical compounds, such as XeF4.
or to say:
Halogens and noble gases are two different groups of elements that can be seen on the periodic table. Halogens are found in group 17 and include fluorine, chlorine, bromine, iodine and astatine. Noble gases make up group 18, and include helium, neon, argon, krypton, xenon and radon.
2. Find the magnetic moment on the following ions Mn2+, Fe2+, Fe3+, Co2+, Ni2+ and Cu2+. If the magnetic moment comes only doe to electrons (orbital contribution is zero), what is the Curie constant for N number of these ions?
Answers
The magnetic moments (in Bohr magnetons) for the ions are: Mn2+ = 5.92, Fe2+ = 4.90, Fe3+ = 5.92, Co2+ = 3.87, Ni2+ = 2.83, Cu2+ = 1.73.
To determine the magnetic moments of the ions, we need to consider the number of unpaired electrons present in each ion. The formula for calculating the magnetic moment due to electron spin is given by:
μ = √(n(n + 2)) * μB
where μ is the magnetic moment, n is the number of unpaired electrons, and μB is the Bohr magneton.
Let's calculate the magnetic moments for each ion:
Mn2+:
Manganese (Mn) has an atomic number of 25, and Mn2+ has 24 electrons. The electron configuration of Mn2+ is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^5.
Since there are 5 unpaired electrons (n = 5), the magnetic moment is:
μ(Mn2+) = √(5(5 + 2)) * μB = 5.92 μB
Fe2+:
Iron (Fe) has an atomic number of 26, and Fe2+ has 24 electrons. The electron configuration of Fe2+ is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6.
Since there are 4 unpaired electrons (n = 4), the magnetic moment is:
μ(Fe2+) = √(4(4 + 2)) * μB = 4.90 μB
Fe3+:
Fe3+ has 23 electrons. The electron configuration of Fe3+ is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^5.
Since there are 5 unpaired electrons (n = 5), the magnetic moment is:
μ(Fe3+) = √(5(5 + 2)) * μB = 5.92 μB
Co2+:
Cobalt (Co) has an atomic number of 27, and Co2+ has 25 electrons. The electron configuration of Co2+ is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^7.
Since there are 3 unpaired electrons (n = 3), the magnetic moment is:
μ(Co2+) = √(3(3 + 2)) * μB = 3.87 μB
Ni2+:
Nickel (Ni) has an atomic number of 28, and Ni2+ has 26 electrons. The electron configuration of Ni2+ is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^8.
Since there are 2 unpaired electrons (n = 2), the magnetic moment is:
μ(Ni2+) = √(2(2 + 2)) * μB = 2.83 μB
Cu2+:
Copper (Cu) has an atomic number of 29, and Cu2+ has 28 electrons. The electron configuration of Cu2+ is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^9.
Since there is 1 unpaired electron (n = 1), the magnetic moment is:
μ(Cu2+) = √(1(1 + 2)) * μB = 1.73 μB
The magnetic moments for the ions are as follows:
Mn2+: 5.92 Bohr magnetons
Fe2+: 4.90 Bohr magnetons
Fe3+: 5.92 Bohr magnetons
Co2+: 3.87 Bohr magnetons
Ni2+: 2.83 Bohr magnetons
Cu2+: 1.73 Bohr magnetons
To calculate the Curie constant for N number of these ions, we need to sum up the magnetic moments for the respective ions and use the formula:
C = (n(n + 2))/3 * μB^2 * μ0
Please note that the above calculations assume that the orbital contribution to the magnetic moment is zero, as specified in the question.
To learn more about magnetic, visit
https://brainly.com/question/30633452
#SPJ11
which of the following drugs blocks the conducting pore of the gaba(a) receptors? a. Benzodiazepines b. Picrotoxin c. Bicuculline d. Strychnine
Answers
The drug that blocks the conducting pore of the GABA(A) receptors is c) Bicuculline.
GABA(A) receptors are ion channels in the central nervous system that mediate the inhibitory effects of the neurotransmitter gamma-aminobutyric acid (GABA). These receptors have a conducting pore through which chloride ions flow when activated by GABA binding.
Benzodiazepines, such as diazepam, enhance the activity of GABA(A) receptors by increasing the affinity of GABA for its binding site, but they do not directly block the conducting pore. Therefore, option a) is incorrect.
Picrotoxin is a noncompetitive antagonist of GABA(A) receptors that acts by binding to a different site on the receptor complex and blocking the chloride ion channel. However, it does not specifically block the conducting pore. Hence, option b) is also incorrect.
Bicuculline is a competitive antagonist of GABA(A) receptors that specifically blocks the conducting pore of the receptor. It binds to the same site as GABA but does not activate the receptor, leading to the inhibition of chloride ion influx. Thus, option c) is the correct answer.
Strychnine is not directly related to GABA(A) receptors and does not block their conducting pore. Therefore, option d) is incorrect.
In summary, among the given options, bicuculline is the drug that blocks the conducting pore of the GABA(A) receptors.
To know more about drug , refer here :
https://brainly.com/question/29767316#
#SPJ11
PLEASE HELP ILL GIVE BRAINLIEST

Answers
how many moles of water are produced in this reaction 2c8h18(g)+25o2(g)→16co2(g)+18h2o(g)
Answers
To determine the number of moles of water produced in the given reaction 2C8H18(g) + 25O2(g) → 16CO2(g) + 18H2O(g), we need to compare the stoichiometric coefficients of water (H2O) with the other reactants and products. From the balanced equation, it can be concluded that 18 moles of water are produced for every 2 moles of C8H18 consumed.
The balanced equation shows that for every 2 moles of C8H18 consumed, 18 moles of H2O are produced. This ratio is obtained by comparing the stoichiometric coefficients of water and C8H18 in the balanced equation.
Therefore, if we have a known amount of C8H18 and want to determine the corresponding moles of water produced, we can use the ratio:
moles of H2O = (moles of C8H18) x (18 moles of H2O / 2 moles of C8H18)
The ratio of 18 moles of H2O to 2 moles of C8H18 indicates that for every 2 moles of C8H18, 18 moles of H2O are produced in the reaction.
Using this ratio, you can calculate the number of moles of water produced by multiplying the number of moles of C8H18 by the ratio of 18 moles of H2O to 2 moles of C8H18.
To learn more about stoichiometric coefficients visit: brainly.com/question/28213872
#SPJ11
Draw the dot and cross diagram for CaCl2.
P.S:I need the answer ASAP
Answers
The dot structure of the compound that is referred to here is shown in the image attached.
What is a dot structure?
A dot structure, often called a Lewis structure or an electron dot structure, depicts how atoms and valence electrons are arranged in a molecule or ion. Around the atomic symbols, valence electrons are represented by dots.
Each atom's symbol in a dot structure is surrounded by dots that stand in for the atom's valence electrons. The electrons in an atom's outermost shell known as valence electrons participate in chemical bonding.
Learn more about dot structure:https://brainly.com/question/28652824
#SPJ1

What are the products of hydrocarbon combustion?
Answers
Answer:
Since hydrocarbon fuels only contain two elements, we always obtain the same two products when they burn. In the equation below methane (CH 4) is being burned. The oxygen will combine with the carbon and the hydrogen in the methane molecule to produce carbon dioxide (CO 2) and water (H 2O).
Explanation:
I Hope This Helps!!!!
if you insert 2.75 grams of co how many grams of H2 are also used?
Answers
The mass of H₂ used in the reaction, given that 2.75 g of CO was inserted is 0.39 grams
How do i determine the mass of H₂ used?The mass of H₂ used in the reaction can be obtained as illustrated below:
Balanced equation:
CO + 2H₂ -> CH₃OH
Molar mass of CO = 28 g/molMass of CO from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 2 × 2 = 4 gFrom the balanced equation above,
28 grams of CO required 4 grams of H₂
Therefore,
2.75 grams of CO will require = (2.75 grams × 4 grams) / 28 grams = 0.39 grams of H₂
Thus, we can conclude that the mass of H₂ used in the reaction is 0.39 grams
Learn more about mass:
https://brainly.com/question/21940152
#SPJ1
In 20 moles of copper (II) phosphate, there are _____ moles of copper ions and _____ moles of oxygen atoms.
(a) 20, 60
(b) 20, 80
(c) 40, 80
(d) 60, 120
(e) 60, 160
Answers
The answer is (e) 60, 160: there are 60 moles of copper ions and 160 moles of oxygen atoms in 20 moles of copper (II) phosphate.
The formula for copper(II) phosphate is Cu3(PO4)2.
To find the number of moles of copper ions in 20 moles of copper (II) phosphate, we must first find the number of moles of copper in one mole of copper (II) phosphate.
We have 3 moles of copper in one mole of copper (II) phosphate.
Therefore, we have:3 x 20 = 60 moles of copper ions
To find the number of moles of oxygen atoms in 20 moles of copper (II) phosphate, we first need to find the total number of oxygen atoms in 20 moles of copper (II) phosphate.
In one mole of copper (II) phosphate, there are 8 oxygen atoms (2 from each phosphate ion).
We have:8 x 20 = 160 oxygen atoms.
So, the answer is (e) 60, 160: there are 60 moles of copper ions and 160 moles of oxygen atoms in 20 moles of copper (II) phosphate.
learn more about moles here:
https://brainly.com/question/30885025
#SPJ11
The best way to increase the amount of a solid solute dissolved in a saturated solution would be to
Answers
Answer:
The solubility of a saturated solution can be increased by increasing the temperature.
Explanation:
Temperature -- Generally, an increase in the temperature of the solution increases the solubility of a solid solute. For example, a greater amount of sugar will dissolve in warm water than in cold water. A few solid solutes, however, are less soluble in warmer solutions.
What is the half-life of a radioisotope if a 50-g sample becomes 25 g after 18
days?
O A. 25 days
O B. 9 days
O C. 50 days
O D. 18 days
Answers
Answer:
18 DAYS IS THE HALF LIFE OF THE RADIOISOTOPE.
Explanation:
Using the formula
Nt = No * (1/2)^t/t1/2
where;
Nt = amount remaining = 25 g
No = Initial amounrt of the radioisotope = 50 g
t = time elapsed = 18 days
t1/2 = half life = unknown
Substitute the values into the equation and obtain the half life of the radioisotope;
Nt = No * (1/2) ^t/t1/2
25 = 50 * (1/2) ^18/t1/2
25 /50 = (1/2)^18/ t1/2
1/2 = (1/2)^18/t1/2
1/2)^1 = (1/2)^18/t1/2
1 = 18/t1/2
t1/2 = 18 days.
So therefore the half life of the radioisotope is 18 days.
Answer:
18 days
Explanation:
Did it on A pex
During which process would the ratio of uranium 238 to lead 206 be used?
Answers
Answer: The ratio of the amounts of U 238 and Pb 206 in a rock sample enables the age of the rock to be estimated using the technique of radiometric dating. It moves back in the periodic table until the isotope falls in the band of stability at Pb 206.
Explanation:
The acid-dissociation constant for chlorous acid (HClO2)(HClO2) is 1.1×10−21.1×10−2.(a)Calculate the concentration of H3O+H3O+ at equilibrium if the initial concentration of HClO2HClO2 is 1.81×10−2 MM .(b)Calculate the concentration of ClO2−ClO2− at equilibrium if the initial concentration of HClO2HClO2 is 1.81×10−2 MM .(c)Calculate the concentration of HClO2HClO2 at equilibrium if the initial concentration of HClO2HClO2 is 1.81×10−2 MM .
Answers
The concentration of H3O+ at equilibrium is 1.33×10−3 M. The concentration of HClO2 at equilibrium is 0.018 M.
As the acid-dissociation constant for chlorous acid (HClO2) is 1.1×10−2 and not 1.1×10−21.1×10−2.
(a) Using the equation for the acid dissociation constant, Ka = [H3O+][ClO2-]/[HClO2], we can rearrange to find the concentration of H3O+ at equilibrium:
Ka = [H3O+][ClO2-]/[HClO2]
1.1×10−2 = [H3O+]^2 / (1.81×10−2)
[H3O+] = sqrt(1.1×10−2 * 1.81×10−2) = 1.33×10−3 M
Therefore, the concentration of H3O+ at equilibrium is 1.33×10−3 M.
(b) Using the equation for the acid dissociation constant, Ka = [H3O+][ClO2-]/[HClO2], we can rearrange to find the concentration of ClO2- at equilibrium:
Ka = [H3O+][ClO2-]/[HClO2]
1.1×10−2 = (1.33×10−3)[ClO2-]/(1.81×10−2)
[ClO2-] = (1.1×10−2)(1.81×10−2) / (1.33×10−3) = 0.149 M
Therefore, the concentration of ClO2- at equilibrium is 0.149 M.
(c) Using the equation for the acid dissociation constant, Ka = [H3O+][ClO2-]/[HClO2], we can rearrange to find the concentration of HClO2 at equilibrium:
Ka = [H3O+][ClO2-]/[HClO2]
1.1×10−2 = (1.33×10−3)(0.149)/[HClO2]
[HClO2] = (1.33×10−3)(0.149) / 1.1×10−2 = 0.018 M
Therefore, the concentration of HClO2 at equilibrium is 0.018 M.
Learn more about equilibrium here:-
https://brainly.com/question/30694482
#SPJ11
Explain how the digestive/excretory system is similar to a recycling center
Answers
Answer:
The digestive system is a system where our body breaks down food to acquire important nutrients. This is the same as your local recycling center because they collect and breaks the plastic down to be reused into recycled products.
The excretory system is a system where we remove excess unnecessary materials. This can be the same as the recycling center because they remove unnecessary materials (such as trash that is not meant to be recycled).
A 6 kg rock rolls down a hill with a momentum of 12 kg m/s. Work out the velocity of the rock.
Answers
momentum = mass x velocity
When know that:
momentum is 12 km m/s
Mass is 6 kg
Hence, velocity = 12/ 6 = 2m/s
Why does ninhydrin stain the skin blue? a. Skin contains amino acids. b. Ninhydrin is blue-colored c. Ninhydrin turns blue when warmed
Answers
Option A, Ninhydrin is a chemical that is used to detect the presence of amino acids in a sample. It reacts with the amino acids in a sample, such as skin, to form a complex that is blue in color.
Ninhydrin is a chemical that is used to detect the presence of amino acids in a sample. It reacts with the amino acids in a sample, such as skin, to form a complex that is blue in color. This is because when Ninhydrin reacts with an amino acid it forms a complex with the nitrogen in the amino group, and this complex is blue in color. It is also commonly used in forensic science to detect fingerprints, as fingerprints contain amino acids from the oils and sweat on the skin. The blue coloration of the skin is an indication of the presence of amino acids, which are found in many biological molecules such as proteins and enzymes.
Learn more about amino acid here:
https://brainly.com/question/24106148
#SPJ4