How many milliliters of sulphur dioxide are formed when 12.5g of iron sulphide ore (pyrite) reacts with oxygen according to the equation at stp?
4fes2+1102=2fe2o3+8so2
pls guys
Answers
4666.7 m of sulphur dioxide are formed when 12.5g of iron sulphide ore (pyrite) reacts with oxygen according to the equation at stp.
According to given data, 12.5 g of iron sulphide ore (Pyrite ) reacts with oxygen according to the equation at STP.
We have to find the volume of sulphur dioxide
Mass of iron sulphide = 12.5 g
molar mass of iron sulphide = 120 g/mol
so number of moles of iron sulphide = 12.5/120 = 0.104167 mol
chemical equation of reaction of iron sulphide with oxygen is given as
4FeS₂ + 11O₂ ⇒2Fe₂O₃ + 8SO₂
here 4 mol of FeS₂ gives 8 mole of sulphur dioxide.
⇒1 mol of FeS₂ = 8/4 mol = 2 mol of sulphur dioxide.
⇒0.104167 of FeS₂ = 2 × 0.104167 = 0.208334 mol of Sulphur dioxide.
at STP 1 mol = 22.4 L
so the mass of sulphur dioxide
= 0.208334 × 22.4 L
= 4.6666816 L
= 4666.6816 ml
≈ 4666.7 ml
Therefore the volume of sulphur dioxide is 4666.7 ml.
To know more about iron sulfide here
https://brainly.com/question/31372347
#SPJ4
Related Questions
Question 2 of 30
A television commercial shows happy people while describing some medical
symptoms. These symptoms include feeling tired and sad. The medication
being advertised by the commercial was approved by the FDA to treat a
disease that causes these symptoms. The narrator says that it is available by
prescription only and contains 1% of the active ingredient. What can you infer
about this medication?
OA. The people in the commercial are happy because they were
treated by the medication.
B. The medication would be more effective if it contained 10% of the
active ingredient.
C. Anyone who has the symptoms should request a prescription
from his or her doctor.
D. The medication can treat people who have the disease described.
Answers
The medication can treat people who have the disease described. According to the commercial, the drug has FDA approval to treat a condition whose symptoms are listed.
Additionally, the narrator notes that the medication only comes with a prescription and has 1% of the active substance. We can deduce from this information that the drug can effectively treat persons who have the condition generating these symptoms, but obtaining it requires a prescription. The commercial provides no support for the other possibilities.
Therefore, the correct option is D.
Learn more about Medication, here:
https://brainly.com/question/11098559
#SPJ1
SOMEONE PLEASE HELP HURRY

Answers
I think the answer is A. I know it's Planet X.
Bài 1. Có 30g dung dịch NaCl 20%. Tính nồng độ % dung dịch thu được khi
a. Pha thêm 20g H2O
b. Cô đặc dung dịch để chỉ còn 25g.
Answers
Answer:
Bài 1. Có 30g dung dịch NaCl 20%. Tính nồng độ % dung dịch thu được khi
a. Pha thêm 20g H2O
b. Cô đặc dung dịch để chỉ còn 25g.
The smallest unit that retains the properties of an element is aan)
atom
O molecule
proton
O compound
neutron
O electron
Answers
Answer:
Atom
Explanation:
An atom is the smallest unit which represents the an element.
It retains the chemical properties of an element.
which of the following chemical reactions represents an acid-base reaction? a. hbr koh ↔ kbr h2o b. nh4oh kcl ↔ koh nh4cl c. zncl2 mgso4 ↔ znso4 mgcl2 d. h2so4 cacl2 ↔ caso4 hcl
Answers
\(NH_4OH KCl\ < - > \ KOH NH_4Cl.\)
This chemical reaction represents an acid-base reaction. The correct answer is (b)
In this reaction, ammonium hydroxide acts as a weak base, while potassium hydroxide acts as a strong base. Hydrochloric acid (HCl) is a strong acid, and ammonium chloride (\(NH_4Cl\)) is a salt formed from the reaction between an acid and a base. The reaction involves the transfer of a proton (H+) from the ammonium hydroxide to the chloride ion. This proton transfer characterizes an acid-base reaction, where a base (\(NH_4OH\)) accepts a proton from an acid (HCl), resulting in the formation of the salt \(NH_4Cl\) and water (\(H_2O\)) as a product. Hence, the correct answer is (b).
To know more about acid-base reaction, here
brainly.com/question/10224396
#SPJ4
the methane generated by usc's wood-chip gasification plant is a
Answers
USC's wood-chip gasification plant generates methane, a clean, renewable energy source, using organic waste like wood chips. This waste-to-energy technology generates syngas, which can be used for electricity and heat, and methanation increases its methane content to 95%.
The methane generated by USC's wood-chip gasification plant is a clean and renewable source of energy. Wood-chip gasification is a type of waste-to-energy technology that uses organic waste, such as wood chips, as fuel to generate electricity, heat, and other forms of energy.
The process involves heating the wood chips in an oxygen-limited environment to produce a gas that is primarily composed of carbon monoxide, hydrogen, and methane. This gas, called syngas, can be used as a fuel to generate electricity and heat.Syngas can be converted into methane through a process called methanation. This process involves adding hydrogen to the syngas, which reacts with the carbon monoxide to produce methane. Methanation can increase the methane content of syngas to around 95%, making it a high-quality fuel that can be used in a variety of applications.
The methane generated by USC's wood-chip gasification plant is a renewable source of energy because it is produced from organic waste that would otherwise be discarded. This means that the plant can generate energy without contributing to the depletion of finite resources such as fossil fuels. Additionally, the use of methane as a fuel produces fewer emissions than traditional fossil fuels, making it a cleaner energy source.
To know more about gasification plant Visit:
https://brainly.com/question/33186105
#SPJ11
in the redox conversion of so3 to so−, s is ? and its oxidation number goes from ? to ?
Answers
The silver atom in this scenario acts as an oxidising agent, whereas the copper atom acts as a reducing agent.
The number of electrons that an element's atoms in a compound loss or gain is known as the oxidation number. The symbol (+) and (-) are typically used before the magnitude when writing oxidation numbers. The oxidation number will be zero if the atoms are present in their elemental form. The oxygen (O) oxidation number in compounds is typically -2. the following compound: Let S be in the oxidation state "x." As a result, sulfur's (S) oxidation number is (+6). Any interaction between an oxidising and a reducing agent is referred to as a redox reaction. In the reaction, one species is oxidised while the other species is reduced. The reduced specie suffers a drop in oxidation number while the oxidation number of the oxidised specie grows.
Learn more about Redox reaction here:
https://brainly.com/question/28207747
#SPJ4
What does it mean for the energy levels in an atom to be quantized? Describe how this affects where electrons can be found.
Answers
Answer:
Energy of an electron is quantinized means the electrons can possess only specific individually separated values.
These energy levels are stable and are stationary along with different energy value. The electron remains in the state until energy is absorbed or released.
The electrons absorb energy when it moves from lower energy state to higher energy state. The electrons lose energy when it moves from higher energy state to lower energy state and thus emit radiations corresponding to the energy gap and is called as quanta.
What is the mass of 6.02 × 10²³ hydrogen atoms
Answers
Answer:
1.0 something depending on your periodic table.
Explanation:
You are given atoms. Not molecules. The mass is what you would read from the periodic table. 1.000 in some form is the answer.
In nature, Hydrogen is found as H2 everywhere except in the sun or a star. The molecular mass (not the atomic mass) would be 2.000 something.
Isotopes have the same number of protons, but a different mass.
True
False
Answers
Answer:
True
Explanation:
they have the same number of protons but different number of neutrons which results in a different mass
I would greatly appreciate if you can give this the brainliest answer crown!
(04.03 LC)
Which of the following is used up during cellular respiration?
O Carbon dioxide
O Mitochondria
O Oxygen
O Water
Answers
Answer:
Oxygen
Explanation:
Cellular respiration is the metabolic process that involves the breakdown of glucose molecule in order to synthesize energy in form of ATP. However, this cellular respiration can either be aerobic or anaerobic depending on whether oxygen is used or not.
Aerobic cellular respiration is the respiration that involves the use of OXYGEN. It occurs in the mitochondria of the cell, where oxygen is used as the final electron acceptor. Hence, according to the question, OXYGEN is used up during cellular respiration.
Answer:
Oxygen
Explanation:
got it on the test(:
the atmospheric pressure in Denver, Colorado, is usually about 84.0 kPa. what is this pressure in atm and torr units?
Answers
- The atmospheric pressure in Denver, Colorado, is approximately 0.829 atm.
- The atmospheric pressure in Denver, Colorado, is approximately 627.522 torr.
The atmospheric pressure in Denver, Colorado, is usually about 84.0 kPa. To convert this pressure to atm and torr units, we can use the following conversion factors:
1 atm = 101.325 kPa
1 atm = 760 torr
First, let's convert 84.0 kPa to atm:
Pressure in atm = 84.0 kPa × (1 atm / 101.325 kPa) ≈ 0.829 atm
Therefore, the atmospheric pressure in Denver, Colorado, is approximately 0.829 atm.
Next, let's convert 84.0 kPa to torr:
Pressure in torr = 84.0 kPa × (760 torr / 101.325 kPa) ≈ 627.522 torr
Therefore, the atmospheric pressure in Denver, Colorado, is approximately 627.522 torr.
For more such information on: atmospheric pressure
https://brainly.com/question/19587559
#SPJ8
Two astronauts are in outer space; hank is inside the space shuttle & the other, sally, is on a space walk. A large meteor hits the space shuttle, but only one of the two astronauts are able to hear it. Which astronaut is able to hear the impact & which isn’t. Explain why. Be sure to include the terms: medium and transfer in your explanation.
Answers
Answer:
Sally would not be able to hear the impact of the meteor because sound waves cannot travel through the vacuum of outer space. In order for sound to be heard, it must vibrate through a medium such as air, water, or solid matter. Since there is no air or other medium in outer space, sound waves cannot transfer through it and cannot be heard. Hank, on the other hand, would be able to hear the impact of the meteor because he is inside the space shuttle, which provides a solid medium for the sound waves to vibrate through and be transferred to his ear drums.
Calculate the average reading for all three trials for both the wet bulb and dry bulb thermometers. Convert the average
temperatures for both thermometers to Celsius using this formula:
(°F - 32) = °C.
Answers
Answer: °32= 0 °C.
Explanation:
Answer:
Explanation:
Please add the temperature readings from all three trials for both thermometers. Will do the calculation with readings.
why should traces of water be removed from organic liquids before running the IR
Answers
The removal of traces of water from organic liquids before IR spectroscopy is important to obtain accurate and reliable results in the analysis of the chemical composition of the sample.
Infrared (IR) spectroscopy is a commonly used analytical technique to identify and quantify the chemical composition of a sample. In order to obtain accurate and reliable results, it is important to remove traces of water from organic liquids before running an IR analysis.
Water has a strong and broad absorption band in the IR region, which can interfere with the spectral analysis of the organic liquid. If water is present in the sample, it will absorb IR radiation in the same frequency range as many of the functional groups found in organic compounds. This can lead to spectral overlap and a reduction in the intensity of the signals for the organic compounds, making it difficult to accurately identify and quantify the chemical composition of the sample.
In addition, the presence of water can also lead to hydrolysis reactions that can alter the chemical composition of the sample and lead to inaccurate results.
To prevent these issues, it is important to thoroughly dry the organic liquid sample before running an IR analysis. This can be done using a drying agent, such as anhydrous magnesium sulfate or sodium sulfate, or by evaporating the water under a stream of dry nitrogen or argon gas.
To know more about Infrared click here:
https://brainly.com/question/20779091#
#SPJ11
what happens when ice changes to water the link up of the atoms
Answers
Answer:
Removing heat causes water (a liquid) to freeze to form ice (a solid). When water changes to a solid or a gas, we say it changes to a different state of matter. ... This causes the hydrogen atoms in one water molecule to be attracted to the oxygen atom in another water molecule.
Explanation:
True or false: Denaturation of an enzyme increases the rate at which it may catalyze a chemical reaction.
Answers
what nacl nacl concentration results when 279 ml 279 ml of a 0.840 m 0.840 m nacl nacl solution is mixed with 442 ml 442 ml of a 0.220 m 0.220 m nacl nacl solution?
Answers
The final NaCl concentration when 279 ml of a 0.840 m NaCl solution is mixed with 442 ml of a 0.220 m NaCl solution is 0.46 m NaCl.
To calculate this, the formula for mixing two solutions of different concentrations is:
C₁V₁ + C₂V₂ = CfVf,
where C₁ and C₂ are the concentrations of each solution, V₁ and V₂ are the volumes of each solution, and Cf and Vf are the final concentration and volume, respectively.
Using the formula, we can calculate the final NaCl concentration to be 0.476 m by solving for Cf.
Cf = (C₁V₁ + C₂V₂) / Vf
Cf = ((0.840 m × 279 ml) + (0.220 m × 442 ml)) / (279 ml + 442 ml)
Cf = 0.476 m NaCl.
Learn more about concentration here: https://brainly.com/question/29457847.
#SPJ11
What is a reactant?
A- Solid particles that have been separated from a solution
B- A substance that has the ability to be dissolved by a solvent
C- A mixture in which a solid and a liquid or two liquids are mixed occurs
D- The substance present before a chemical reaction occurs
Answers
Answer:
it is D
Explanation:
because it is a substance that participants in a chemical reaction.
89. What is the pressure of a fixed volume of a gas at 30.0C if it has a pressure of 1.11atm at 15C?
Answers
0.555 atm is the pressure of a fixed volume of a gas at 30.0C if it has a pressure of 1.11atm at 15C.
What is an ideal gas equation?The ideal gas equation, pV = nRT, is an equation used to calculate either the pressure, volume, temperature or number of moles of a gas.
According to Ideal Gas Equation,
PV = nRT
Therefore, P ∝ T
P₁T₂ = P₂T₁
Given :
P₁ = ?
P₂ = 1.11 atm
T₁ = 30.0 C
T₂ = 15 C
Solving :
P₁ x 30 = 1.11 x 15
P₂ = 0.555 atm
Hence, 0.555 atm is the pressure of a fixed volume of a gas at 30.0C if it has a pressure of 1.11atm at 15C.
Learn more about ideal gas equation here:
https://brainly.com/question/14826347
#SPJ2
correct way for calculating atomic mass
Answers
Answer:
mass number = protons + neutrons.
Explanation:
Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
Among the types of radiation, alpha, beta, gamma, and x-ray, the one that requires the least amount of protective the clothing is _______ radiation
Answers
Among the types of radiation, alpha, beta, gamma, and x-ray, the one that requires the least amount of protective clothing is x-ray radiation.
X-rays are a form of electromagnetic radiation, and they have a shorter wavelength than gamma rays, which means that they carry less energy. As a result, they are less harmful to living tissues and do not require as much protection. In many cases, only a lead apron or a lead vest is needed to shield the patient from x-rays during medical procedures.
Alpha and beta radiation, on the other hand, are particles that can cause significant damage to living tissues and require more extensive protective measures, such as gloves, masks, and full-body suits. Gamma radiation is also highly penetrative and requires thick layers of lead or concrete to shield against it.
In summary, x-ray radiation is the type of radiation that requires the least amount of protective clothing due to its lower energy and shorter wavelength compared to other types of radiation. However, it is still important to take appropriate safety precautions when working with x-rays to minimize exposure and potential harm.
learn more about radiation
https://brainly.com/question/31476599
#SPJ11
the distance between carbon atoms in ethylene is 134 picometers. which of the following expresses that distance in meters?
Answers
To convert picometers to meters, we need to divide the distance by 10^12 (1 trillion). So, 134 picometers can be expressed as 134/10^12 meters. In scientific notation, this would be 1.34 x 10^-10 meters.
It's important to note that the distance between carbon atoms in ethylene is crucial to understanding the chemical and physical properties of this molecule. Ethylene is a hydrocarbon, meaning it consists of only carbon and hydrogen atoms. The distance between the two carbon atoms in the molecule determines its overall shape and reactivity.
For example, the double bond between the two carbon atoms in ethylene allows for the molecule to undergo addition reactions with other molecules. This reactivity is important in industrial processes such as polymerization, where ethylene is used to create plastic materials.
Furthermore, the distance between carbon atoms in ethylene is also important in understanding its physical properties. The molecule has a low boiling point due to the weak intermolecular forces between the molecules. This is because the carbon-carbon bond length is relatively short, leading to a compact and less polar molecule.
Overall, the distance between carbon atoms in ethylene may seem like a small detail, but it has significant implications for the chemistry and properties of this molecule.
To know more about Carbon visit:
https://brainly.com/question/26789123
#SPJ11
HELPPPP!!!! SCIENCE!!!!
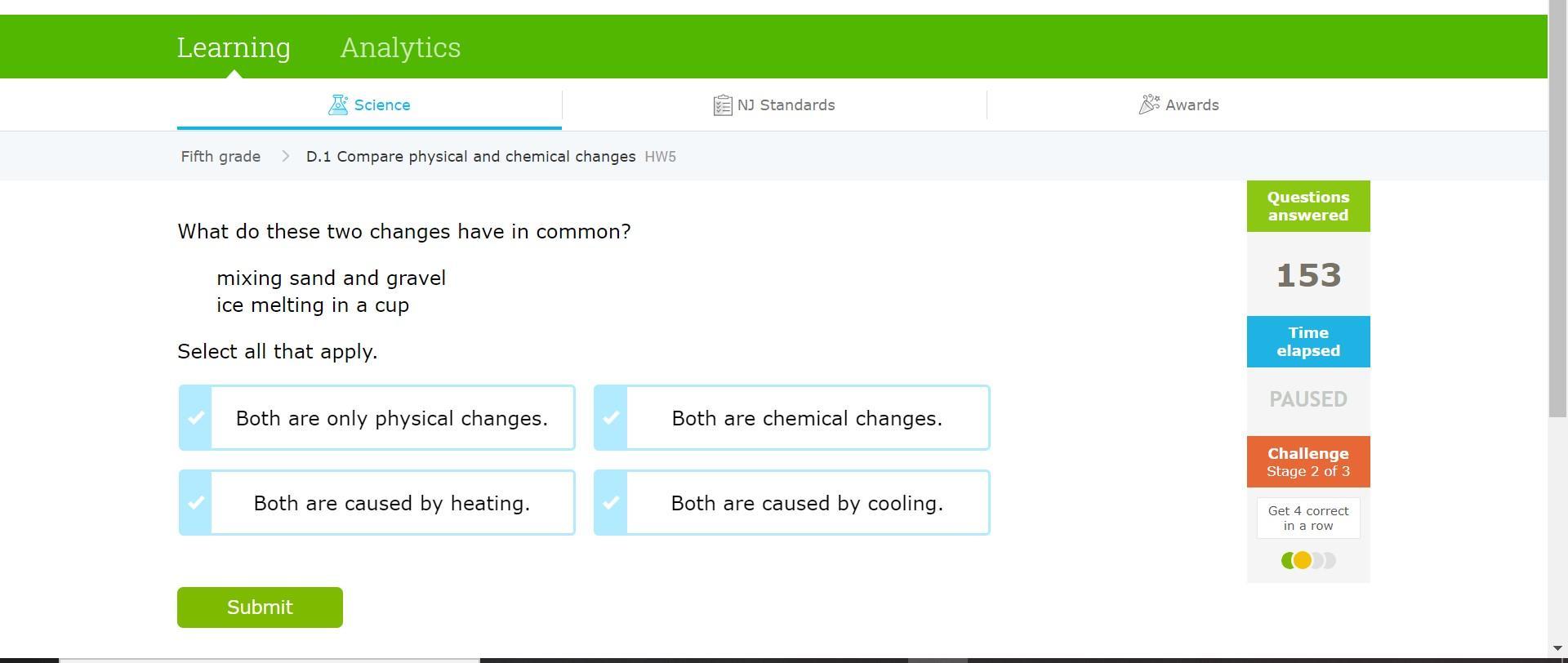
Answers
Answer:
Both are only physical changes
Germanium has 5 isotopes. The average atomic mass from the periodic table is 72.63.
A)Find the missing abundance.
B) Use the atomic mass to calculate the mass of the missing isotope.

Answers
Answer:
75.92
Explanation:
A)the % abundance adds up to 100, so adding all the % abundances of Ge:
20.52 + 27.45 + 7.76 + 36.52 = 92.25
The remaining % abundance is:
100 - 92.25 = 7.75%
B) Using this formula:
\(atomic \: mass = \frac{sum \: of \: (\% \: isotope \: abundance \times isotope \: mass)}{100} \)
\(72.63 = \frac{(20.52 \times 69.92) + (27.45 \times 71.93) + (7.76 \times 72.92) + (36.52 \times 73.92) + (7.75x)}{100} \)
multiply both sides by 100 to get rid of the fraction:
\(7263= {(20.52 \times 69.92) + (27.45 \times 71.93) + (7.76 \times 72.92) + (36.52 \times 73.92) + (7.75x)}{} \)
multiplying each bracket:
\(7263 = 1434.7584 + 1974.4785 + 565.8592 + 2699.5584 + 7.75x\)
adding up the terms:
\(7263 = 6674.6545 + 7.75x\)
subtract 6674.6545 on both sides to give:
\(588.3455 = 7.75x\)
divide both sides by 7.75:
\(x = \frac{588.3455}{7.75} = 75.92 \: (2dp)\)
Li2O + MgCl2 → 2 LiCl + MgO
What type of reaction is going on in this equation.
Answers
Answer:
water
Explanation:
The esterification reaction is carried out by removing water azeotropically. Why can't calciu chloride pellets be used instead to remove water? Explain.
Answers
Because calcium chloride pellets absorb moisture from the air rather than the reaction mixture, they cannot be employed to remove water from the esterification reaction.
Desiccant calcium chloride pellets are routinely used to absorb moisture from the air. They cannot, however, be utilized to remove water from an esterification reaction because their water absorption is not selective.
It means that, calcium pellets will absorb the moisture from the surroundings including the air. In contrast, azeotropic distillation employs a solvent to generate an azeotrope with water that can be removed from the reaction mixture, essentially removing water from the reaction.
To know more about esterification reaction, visit,
https://brainly.com/question/28118164
#SPJ4
Tear off a small, flat sheet of waxed paper. Use the pipette to dispense a drop of water on the waxed paper. Now, dry the pipette, and use it to dispense a drop of oil on the waxed paper next to the water drop. Be sure the two drops are not touching. Compare and describe the appearance of both drops.
Answers
Answer:
Explanation:
When comparing the drops of oil and water, one thing I noticed was the shape. The water drop was more defined, whereas the drop of oil began to spread and was much flatter. This may be due to the waxy material, and how both oil and water react to the wax.
using the structural formula of the following substances, explain which ones can exist as cis-trans isomers: 1- chloropropane, 2-chloropropane, 1-pentene, 2-pentene
Answers
Cis-trans isomers are a subtype of geometric isomers in which two similar groups on the same atom are arranged differently in space. In the cis form, the two groups are on the same side of the molecule, whereas in the trans form, they are on opposing sides.
Using the structural formula of the following substances, we need to explain which ones can exist as cis-trans isomers:1-chloropropane: CH3CH2CH2Cl can have cis-trans isomers because the Cl atom is attached to the second carbon atom, and there are two different groups attached to that carbon atom.2-chloropropane: CH3CHClCH3 can have cis-trans isomers because the Cl atom is attached to the second carbon atom, and there are two different groups attached to that carbon atom.
1-pentene: CH3CH2CH=CHCH3 can have cis-trans isomers because there is a double bond between the 3rd and 4th carbon atoms, and there are two different groups attached to the carbon atoms adjacent to the double bond.2-pentene: CH3CH=CHCH2CH3 can have cis-trans isomers because there is a double bond between the 2nd and 3rd carbon atoms, and there are two different groups attached to the carbon atoms adjacent to the double bond.
learn more about isomers here
https://brainly.com/question/26298707
#SPJ11
What is the mass of 3.5 moles of silver acetate
[AgCH,COo]?