Answers
mass = mol no. x molar mass
= 13.6 x 56+(3x14)+(9x16)
= 242g
The mass of nitrogen will be 242 gram.
What is gram?The gram as it was originally known, would be a unit of matter in the metric measurements.
What is mass?The quantity of matter in a thing is measured by its mass. Typically, mass is expressed in kilogram (kg) or gram (g) .
Calculation of mass:
Mass can be calculated by using the formula.
Mass = mol × molar mass
Mass = 13.6 × 56 +(3× 14) +(9 × 16).
Mass = 242 g.
Therefore, the mass of nitrogen will be 242 gram.
To know about mass and gram
https://brainly.com/question/19694949
#SPJ3
Related Questions
Which solids are insoluble in water.
Answers
Some types of solids that are insoluble in water are:
Metals. (most of them)Non-Metallic ElementsMetal OxidesSome Non-Metallic ElementsMetal Carbonates (most of them)Metal Sulfides (most of them)Salts (some of them)Which solids are insoluble in water?Many solids are insoluble in water, meaning they do not dissolve in water to a significant extent. Here are some examples of common solids that are generally insoluble in water:
Metals: Most metals, such as gold, silver, platinum, and copper, are insoluble in water.
Non-Metallic Elements: Many non-metallic elements, such as carbon (in the form of graphite or diamond), sulfur, phosphorus, and iodine, are insoluble in water.
Metal Oxides: Some metal oxides, particularly those of less reactive metals, are insoluble in water. Examples include aluminum oxide (Al2O3), iron(III) oxide (Fe2O3), and lead(II) oxide (PbO).
Metal Carbonates: Most metal carbonates are insoluble in water. Examples include calcium carbonate (CaCO3), lead(II) carbonate (PbCO3), and copper(II) carbonate (CuCO3).
Metal Sulfides: Many metal sulfides are insoluble in water. Examples include lead(II) sulfide (PbS), silver sulfide (Ag2S), and mercury(II) sulfide (HgS).
Insoluble Salts: Certain salts have limited solubility in water. Examples include silver chloride (AgCl), lead(II) iodide (PbI2), and calcium sulfate (CaSO4).
It's important to note that while these solids are generally insoluble in water, they may exhibit some solubility to a small extent. The solubility of a solid in water can vary depending on factors such as temperature, pressure, and the presence of other solutes.
Learn more about solubility:
https://brainly.com/question/23946616
#SPJ1
A 5.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 78./gmol, is burned completely in excess oxygen, and the mass of the products carefully measured:
product mass
carbon dioxide 16.93g
water 3.46g
Use this information to find the molecular formula of X.
Answers
Answer:
C6H6
Explanation:
Step 1:
Data obtained from the question. This include the following:
Mass of the compound = 5g
Molar mass of the compound = 78g/mol
Mass of CO2 = 16.93g
Mass of H2O = 3.46g
Step 2:
Determination of the mass of carbon, hydrogen and oxygen present ij the compound. This can be obtained as follow:
Molar mass of CO2 = 12 + (2x16) = 44g/mol
Mass of C = 12/44 x 16.93 = 4.62g
Molar mass of H2O = (2x1) + 16 = 18g/mol
Mass of H = 2/18 x 3.46 = 0.38g
Mass of O = 5 – (4.62 + 0.38) = 0
Step 3:
Determination of the empirical formula of the compound.
C = 4.62g
H = 0.38g
Divide by their molar mass
C = 4.62/12 = 0.385
H = 0.38/1 = 0.38
Divide by the smallest
C = 0.385/0.38 = 1
H = 0.38/0.38 = 1
Therefore, the empirical formula of the compound is CH.
Step 4:
Determination of the molecular formula of the compound.
The molecular formula of a compound is simply a multiple of the empirical formula.
The molecular formula => [CH]n
[CH]n = 78
[12 + 1]n = 78
13n = 78
Divide both side by 13
n = 78/13
n = 6
The molecular formula => [CH]n
=> [CH]6
=> C6H6.
Therefore, the molecular formula of the compound is C6H6.
How many moles are in 1.5 x 1022 atoms of Cu?
Answers
There are 0.0249 moles of Cu in 1.5 x 10²² atoms of Cu.
What is Mole?
A mole is a unit of measurement used in chemistry to express amounts of a chemical substance. It is defined as the amount of a substance that contains the same number of entities (such as atoms, molecules, or ions) as there are in 12 grams of pure carbon-12, which is approximately 6.022 x 10²³ entities.
This number is called Avogadro's number and is used as a conversion factor between the mass of a substance and the number of particles it contains.
To calculate the number of moles, we need to know the atomic mass of Cu, which is 63.55 g/mol.
We can use Avogadro's number, 6.022 x 10²³, to convert the number of atoms to moles:
1.5 x 10²² atoms Cu * (1 mol Cu / 6.022 x 10²³ atoms Cu) = 0.0249 mol Cu
Therefore, there are 0.0249 moles of Cu in 1.5 x 10²² atoms of Cu.
Learn more about Mole from given link
https://brainly.com/question/29367909
#SPJ1
There are 0.0249 moles of Cu in 1.5 x 10^22 atoms of Cu.
What is Mole?
A mole is a unit of measurement used in chemistry to express amounts of a chemical substance. It is defined as the amount of a substance that contains the same number of entities (such as atoms, molecules, or ions) as there are in 12 grams of pure carbon-12, which is approximately 6.022 x 10^23 entities. This number is called Avogadro's number and is used as a conversion factor between the mass of a substance and the number of particles it contains.
To calculate the number of moles, we need to know the atomic mass of Cu, which is 63.55 g/mol.
We can use Avogadro's number, 6.022 x 10^23, to convert the number of atoms to moles:
1.5 x 10^22 atoms Cu * (1 mol Cu / 6.022 x 10^23 atoms Cu) = 0.0249 mol Cu
Therefore, there are 0.0249 moles of Cu in 1.5 x 10^22 atoms of Cu.
Learn more about Mole from given link
https://brainly.com/question/24191825
#SPJ1
Convert 0.672ft to millimeters. This is the chart given

Answers
Answer: 204.8256 millimeters
Explanation:
Why at night, under the mercury or sodium vapor lights in a mall parking lot, do cars seem to be peculiar colors?3
Answers
Answer:
Because these lights work with a combination of gases that emits a yellow-orange light that reacts with the paint of cars.
Explanation:
The mercury or sodium vapor lights work with a very interesting gas structure that when reacting with each other, emit yellow-orange lights that react with the color of the cars making them appear to have other colors.
When these lights are placed in parking lots, the white cars will turn orange. This is because the white paint used in cars contains particles that emit a yellow-orange spectrum. However, cars of other colors do not have these particles and therefore become black under these lights.
Your bus is traveling 80 km/hr on a class trip to a museum that is 240 kilometers from your school. How long will it take to get there?
Answers
I need help figuring it out the answers were wrong I put in

Answers
A student runs 7km in 2 hours to train for sports.what is the students average speed
Answers
The students average speed of the student is 3.5 km/h.
What is speed?Speed is known to be calculated as the ratio of distance traveled and time traveled. It is a scalar quantity since it only has magnitude and not direction. Velocity is the percentage of time an object moves along a path, and Velocity is expressed as the rate and direction of an object's movement.
The mathematical calculation of speed is relatively simple, calculating the average velocity of an object by dividing the distance traveled by the time it took the object to travel that distance. Velocity, on the other hand, is mathematically complex and can be calculated in different ways depending on what information is available about the object's motion.
Speed = Distance/Time
Speed = 7km/2h
Speed = 3.5 km/h
To know more about average speed, visit:
https://brainly.com/question/6280317
#SPJ1
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
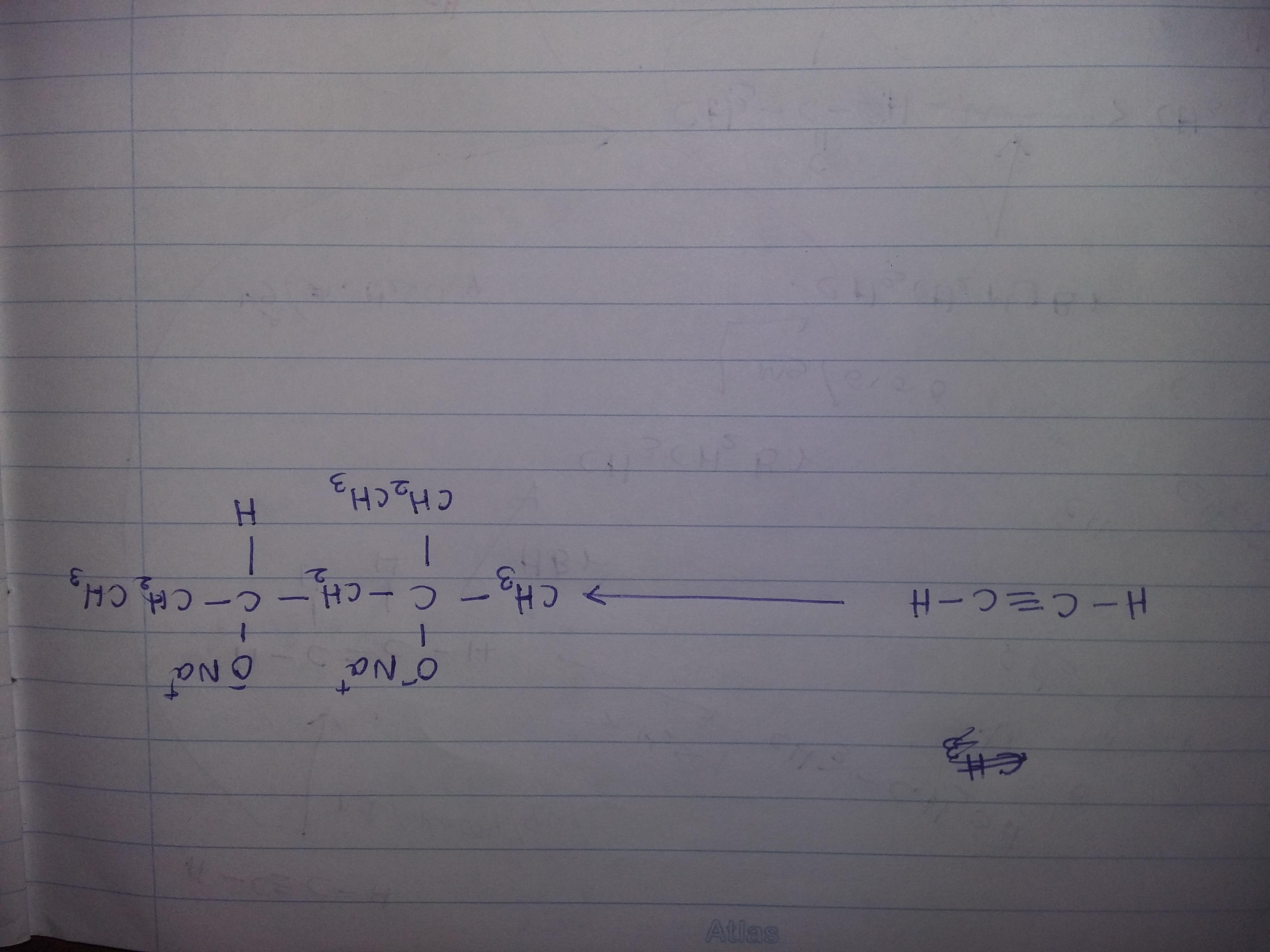
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
two uses of sodium carbonate
Answers
Sodium carbonate, also known as washing soda or soda ash, has a wide range of applications. Sodium carbonate can be naturally occurring or synthetically produced through various methods, including the Solvay process, which is the most common method of industrial production.
Sodium carbonate, also known as washing soda or soda ash, has many uses, including:
1) Cleaning agent: Sodium carbonate is an effective cleaning agent due to its alkaline nature. It is used in laundry detergents and household cleaners to remove stains and grease from clothes and surfaces.
2) Industrial applications: Sodium carbonate is used in a variety of industrial applications. It is used in the production of glass, pulp and paper, and soaps and detergents. It is also used as a water softener and pH regulator in chemical processes.
Learn more about Sodium Carbonate at
brainly.com/question/31344166
#SPJ1
How does the specific heat of water affect the oceans?
A. It keeps the ocean temperatures more even.
B. It causes the rise and fall of tidewater.
C. It keeps the ocean water from freezing.
D. It increases the conductivity of seawater.
Answers
The specific heat capacity of water is 4.18 J/g*C which is on the high side, this implies that it will take a high amount of heat for water to get heated sufficiently.
A. It keeps the ocean temperatures more even.
Heat capacity of waterThe heat capacity of a substance can be defined as the quantity of heat needed to raise a unit mass of that substance by a unit change in its temperature
Learn more about heat capacity here:
https://brainly.com/question/21406849
Answer: A
Explanation:
i just took the test and got it right
What is the pH of a solution that contains 2.1 x 10-10 M hydronium ion concentration?
Answers
please help with this

Answers
Answer:
A sample of a gas (5.0 mol) at 1.0 atm is expanded at constant temperature from 10 L to 15 L. The final pressure is 0.67 atm.
Step by Step Explanation?
Boyle's law states that in constant temperature the variation volume of gas is inversely proportional to the applied pressure.
The formula is,
P₁ x V₁ = P₂ × V₂
Where,
P₁ is initial pressure = 1 atm
P2 is final pressure = ? (Not Known)
V₁ is initial volume = 10 L
V₂ is final volume = 15 L
Now put the values in the formula,
\begin{gathered}\rm 1\times 10 = P_2\times 15\\\\\rm P_2 = \frac{10}{15\\} \\\\\rm P_2 = 0.67\end{gathered]
Therefore, the answer is 0.67 atm.
How does the burning of fossil fuels contribute to global warming?
Answers
Answer: The burning of fossil fuels releases carbon dioxide, methane and other greenhouse gases into the atmosphere. These gases trap heat in the atmosphere, leading to a gradual increase in global average temperatures, known as global warming. This phenomenon has serious impacts on our environment and ecosystems, including extreme weather events and rising sea levels.
Which statement is correct? A. When the concentration of the reactant decreases, the cell potential increases. B. When the concentration of the reactant decreases, the cell potential does not change. C. When the concentration of the reactant decreases, the cell potential decreases. D. When the concentration of the reactant increases, the cell potential decreases. E. When the concentration of the reactant increases, the cell potential does not change.
Answers
The correct statement is C. When the concentration of the reactant decreases, the cell potential decreases.
The cell potential, also known as the electromotive force or voltage, is a measure of the tendency of a redox reaction to occur. It is directly related to the concentrations of the reactants and products involved in the reaction. According to the Nernst equation, which describes the relationship between cell potential and reactant concentrations, a decrease in the concentration of a reactant leads to a decrease in the cell potential. In a redox reaction, the reactants undergo oxidation and reduction processes. The reactant that is being oxidized is called the oxidizing agent, while the reactant that is being reduced is called the reducing agent. The concentrations of these species affect the reaction rates and, subsequently, the cell potential. When the concentration of the reactant decreases, the reaction rate of the oxidizing agent or the reducing agent decreases. This imbalance in reaction rates disrupts the equilibrium of the redox reaction, resulting in a decrease in the overall cell potential. This decrease occurs because there is less availability of the reactant to participate in the reaction and contribute to the electron transfer process. It is important to note that the concentration of the reactant on its own does not determine the cell potential. Other factors such as the nature of the reactants, temperature, pressure, and electrode potentials also influence the overall cell potential. However, in isolation, when the concentration of the reactant decreases, the cell potential will generally decrease as well.
For such more questions on concentration
https://brainly.com/question/29661329
#SPJ11
What is the mass of 1.78 moles of O2
Answers
Answer:
56.96 grams
Explanation:
To find the mass of 1.78 moles of O2, we need to use the molar mass of O2, which is the mass of one mole of O2.
The chemical formula for O2 is O-O or simply O2. The molar mass of O2 is the sum of the atomic masses of two oxygen atoms, which can be found on the periodic table.
The atomic mass of oxygen (O) is approximately 16.00 g/mol. So the molar mass of O2 is:
Molar mass of O2 = 2 x atomic mass of O
= 2 x 16.00 g/mol
= 32.00 g/mol
Therefore, the mass of 1.78 moles of O2 is:
Mass = number of moles × molar mass
= 1.78 mol × 32.00 g/mol
= 56.96 g
So the mass of 1.78 moles of O2 is 56.96 grams.
Which observation most likely indicates that only a chemical change has taken place
A.States of matter change
B. The change cannot be reversed(my answer)
C. A reaction occurs
D. The shape changes
Answers
The correct answer as to which observation most likely indicates that only a chemical change has taken place would that the change cannot be reversed.
When it comes to changes in a system, it can either be:
physical changechemical changeWhen a substance undergoes a physical change, the original version of the substance can be recovered. In other words, physical changes can be reversible.
When a substance undergoes a chemical change, the original version cannot be recovered because an entirely new product would have been formed. In other words, chemical changes are irreversible.
Thus, once a change becomes irreversible, such a change is said to be a chemical change.
More on chemical change can be found here: https://brainly.com/question/1161517
Answer: A reaction occurs
Explanation:

c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
What is the boiling point of a solution that contains 50.0 g of FeBr3 in 200.0 g of water?
Answers
The answer is 100.431 °C.
we know that we can find the boiling point using the following equation:
ΔT=kb×m
where
ΔT=boiling point elevation
kb=molal boiling point elevation constant (0.51 °C/m for water)
m=molality of the solution
To calculate the molality of the given solution
The molar mass of FeBr3:
Fe:1×55.85g/mol =55.85g/mol
Br:3×79.90 g/mol=239.7 g/mol
Total:295.55 g/mol
number of moles present in 50g is:
n=mass/molar mass=50.0g/295.55g/mol
=0.169mol
mass of water=200.0g
molality=moles of solute/kg of solvent
molality=0.169mol/0.200kg=0.845mol/kg
Finally, we can calculate the boiling point elevation
ΔT=kb×m
ΔT=0.51 °C/m×0.845mol/kg=0.431 °C.
boiling point at standard pressure=100.0 °C.
boiling point of solution=100.0 °C +0.431 °C=100.431 °C
So the answer is 100.431 °C
To know more about Boiling point:
https://brainly.com/question/29233996
help someone i need this

Answers
12.0 grams of sodium reacts with 5.00 grams of chlorine. What mass of sodium chloride could be produced?
Please show work if you can. I have the limiting reactant which is Cl2 (0.0705)
I think I did this part correctly but I don't know what to do after. How do you determine the amount of sodium chloride that can be produced?
Answers
Answer❣
The required speech is as follows :
_______________
"Whoever controls media, controls mind". A very hearty Good Morning to one and all present here ! Respected Principal Sir, worthy teachers and all my dear fellow mates. Today I Aurora, of class XYZ is standing before you in this frizzy winter to deliver a short speech on the topic 'How media influences public opinion'.
Dear friends, first of all I would like to ask you all a question, 'What do you think is our media for ?' Most of us will reply that they are for our entertainment, fun and even unfortunately some will reply 'for movies and Bollywood'. Now if we look back at our history during 18th century we see that even first newspapers were started for spreading information in mass. Earlier literacy rate of India was very low.
A single person used to first read the newspaper and then shared the information to the gathered people around. But that time was different. Now nearly every person has a mobile phone, TV, PC and what not. Has this increased public participation in Democracy ? Yes indeed it has done that. On one single click we get information of our surrounding at a faster pace. As a fourth pillar of Democracy it makes our mindful of different social, political and financial exercises around us.
If we take current situation, most of the election popularisation works in different states is being managed by mass media only due to its advancements. Media makes participation of people in political activities even at grass - root levels. This efficiency enhances the Federalism of India. Media has many roles in shaping public opinion in our society. Let's discuss them one by one. In economic terms, media shows the expenditure, budgets and taxes to the citizens of a nation.
It makes people aware of the different types of money related issues that they have to face. Even it checks that no people are harassed by fake news on balances. Its makes people rise over their concern directly to the government so that actions can be taken for them. In social terms, media promotes the functioning of the government. It helps people to convey their social problems to the government. Even many NGOs and Human Rights Organizations take help of medias to make people aware of their rights.
Commonly Media acts as an inference or better to say 'compass' which gives direction to the views of people. It organises the vast multitudes of opinions into a single issue which can be beneficial to all the groups of society. It works 24 × 7 and makes people get in touch with latest developments. As 'Opposition' in Politics, media acts as a hub where the opposition party by taking views of public claims the wrong decisions of Ruling Party.
Also, during elections media shows mirror about different parties to people so that we can make right decision in voting. All these were some positive aspects of media's influence on public opinions. Now let's discuss about some negative aspects also. Nowadays its a common seen trend that media enlarges a short issue just to gain popularity. This results in riots, violence, protests, etc. because the issue which could have been solved by a single subtle discussion, now has been made a larger issue.
Let me present an example. I have a friend, Cupicake. She remains quite much familiar with medias. Some days ago, she received a post which asked her to share the image attached with it to her other friends. That image was of a case where Police was beating a few groups of people. She has shared that. This led a immediate violence in that region and finally it was known that Police was beating a few robbers. Now who to put blame of that riot ? Of course we are the one who done it.
At last I would like to conclude my speech by just saying that, it is the duty of media to transfer right and reliable information to people and its duty of people to share and trust upon right information from a good source. In this way the world and the nation would be leaded to right path.
Thank you one and all.
_______________
★ More to know :-
Format of Speech :
*Note - Here I used name as Aurora. You can use any according to your choice and class also according to the situation.
✌✌✌✌✌✌✌✌✌✌✌✌
hope that u r helpful
please follow thank and brinlylist please
Thank You...♡♡
a piece of copper weighing 850 grams is placed in a cup with 450 ml of water at 21 C and the Cp of the cup is 47 J/K, how many grams of gasoline would it take to heat the entire system to 110 C?
Answers
Answer:
4.2g of gasoline
Explanation:
In the problem, you need to give energy to the cup from the combustion of gasoline. The energy you need to give is:
Qcup + QWater + QCopper
As you need to increase (110ºC - 21ºC = 89º = Increase 89K) 89K, the Qcup is:
Qcup = 89K × (47J/K) = 4183J.
You can find Qwater using its specific heat, C (4.18Jg⁻¹K⁻¹), its mass (450mL = 450g) and the change of temperature, 89K:
QWater = CₓmₓΔT
QWater = 4.184Jg⁻¹K⁻¹ ₓ 450g×89K
QWater = 167569J
And Q of Copper, QCu, could be obtained in the same way (Specific heat Cu: 0.387 J/g⁻¹K⁻¹:
QCu = CₓmₓΔT
QCu = 0.387 J/g⁻¹K⁻¹ₓ850gₓ89K
QCu = 29277J
Thus, total heat you need is:
Q = Qcup + QWater + QCopper
Q = 4183J + 167569J + 29277J
Q = 201029J = 201kJ
The combustion of gasoline (Octane) produce 47.8kJ/g (Its heat of combustion). that means to produce 201kJ of energy you require:
201kJ × (1g / 47.8kJ) =
4.2g of octane = Gasoline you requireFor the reaction C + 2H2 - CH4
how many grams of carbon are required to produce 10.7 moles of methane, CH4?
Use the following molar masses:
hydrogen: 1
carbon: 12
Answers
Taking into account the reaction stoichiometry, 128.4 grams of C are required to produce 10.7 moles of methane.
Reaction stoichiometryIn first place, the balanced reaction is:
C + 2 H₂ → CH₄
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
C: 1 moleH₂: 2 molesCH₄: 1 moleThe molar mass of the compounds is:
C: 12 g/moleH₂: 2 g/moleCH₄: 16 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
C: 1 mole ×12 g/mole= 12 gramsH₂: 2 moles ×2 g/mole= 4 gramsCH₄: 1 mole ×16 g/mole= 16 gramsMass of C requiredThe following rule of three can be applied: If by reaction stoichiometry 1 mole of CH₄ is produced by 12 grams of C, 10.7 moles of CH₄ are produced by how much mass of C?
mass of C= (10.7 moles of CH₄×12 grams of C)÷1 mole of CH₄
mass of C= 128.4 grams
Finally, 128.4 grams of C are required.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
It is one of the factors affecting metamorphic rock which creates lineation.
Answers
Answer:
Pressures and temperatures
Explanation:
In deciding the new substances that develop in a metamorphic stone, pressures and temperatures are major factors. Under various conditions of pressure and temperature, various minerals form.
Metamorphic rocks are classified that, extreme heat and strain, have altered shape. Metamorphic derives from the terms meta and morph in german. Meta means alteration and morph means shape. So, to alter shape, we get metamorphosed significance.
How many moles of NaHCO3 are in 2.4 x
1024 molecules of NaHCO:?
Answers
4.0 moles NaHCO
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
MolesAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
[Given] 2.4 × 10²⁴ molecules NaHCO
[Solve] moles NaHCO
Step 2: Identify Conversion
Avogadro's Number
Step 3: Convert
[DA] Set up: \(\displaystyle 2.4 \cdot 10^{24} \ molecules \ NaHCO(\frac{1 \ mol \ NaHCO}{6.022 \cdot 10^{23} \ molecules \ NaHCO})\)[DA] Divide [Cancel out units]: \(\displaystyle 3.98539 \ moles \ NaHCO\)Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
3.98539 moles NaHCO ≈ 4.0 moles NaHCO
A scientist is studying two types of worms. Worm X has DNA identical to its parent. Worm Y has a combination of DNA from two parents. The scientist determines that one of the worms has a survival advantage over the other worm. Which statement correctly summarizes this conclusion? Worm X reproduces asexually, which gives it genetic variation. Worm Y reproduces sexually, which gives it genetic variation. Worm Y reproduces asexually, which gives it more DNA. Worm X reproduces sexually, which gives it more DNA.
Answers
Answer:
2. Worm Y reproduces sexually, which gives it genetic variation.
Answer:
B Worm Y reproduces sexually, which gives it genetic variation.
Explanation:
hope you all have a good day
Which of the following is least like the others on the list?
a. Amino acid
b. Ribonucleic acid
c. Nucleic acid
d. Nucleotides
Answers
Explanation:
C option i think but I didn't know
HOW DOES WALTER WHITE SLEEP AT NIGHT
Answers
Answer:
Since Walter can alter time and space he doesn't have the kind of place to take a nap or buy a cap for his dog zap
Explanation:
Answer:
Breaking Bad
Explanation:
walt
Please solve the Problems below and round to the correct sig figs:
1. 0.961 - 0.07
2. 0.961 - 0.0700
3. 540.0 X 2.02
4. 540 x 2.02
Answers
Answer:
1. 0.891
2. 0.891
3. 1,090.8
4. 1,090.8
Explanation: