how many grams of magnesium must react with phosphoric acid to produce 100 L of hydrogen gas
Answers
The amount, in grams, of magnesium that must react with phosphoric acid to produce 100 L of hydrogen gas would be 108.38 grams.
Stoichiometric problemMagnesium reacts with phosphoric acid to produce hydrogen gas and magnesium phosphate according to the following balanced equation:
\(3Mg + 2H_3PO_4 -- > Mg_3(PO_4)_2 + 3H_2\)
The mole ratio of the magnesium that reacts to the hydrogen gas that is produced is 1:1.
If, 1 mole of a gas = 22.4 L, then 100 L of hydrogen gas would be equivalent to:
100/22.4 = 4.46 mols
Since the mole ratio is 1:1, the mole of magnesium that will produce 100 L of hydrogen gas will also be 4.46 moles.
Mass of 4.46 moles magneisum = 4.46 x 24.3
= 108.38 grams
In other words, the amount of magnesium that must react with phosphoric acid in order to produce 100 L of hydrogen gas would be 108.38 grams.
More on stoichiometric problems can be found here: https://brainly.com/question/15047541
#SPJ1
Related Questions
if hcl dissolves exothermically: how does a decrease in temperature affect its solubility? how does an increase in temperature affect its solubility?
Answers
If HCl dissolves exothermically, a decrease in temperature increases solubility, while an increase in temperature decreases solubility.
How does temperature affect the solubility of HCl when it dissolves exothermically?If HCl dissolves exothermically, a decrease in temperature would increase the solubility of the compound, while an increase in temperature would decrease the solubility. This is because solubility is typically favored at lower temperatures for exothermic processes, while an increase in temperature will tend to shift the equilibrium towards the dissociated state of the compound.
The reason for this can be explained by Le Chatelier's principle, which states that a system at equilibrium will adjust its response to counteract any changes in its conditions. When HCl dissolves exothermically, heat is released as the compound dissolves, and so a decrease in temperature would be seen as a shift in the conditions that favor the dissolving of HCl. The system would then respond by shifting the equilibrium towards the side of the reaction that absorbs heat, which is the dissolution of HCl. This would result in an increase in solubility.
Conversely, when the temperature is increased, the system responds by shifting the equilibrium towards the side of the reaction that releases heat, which in this case is the undissociated state of HCl. As a result, the solubility of HCl would decrease with an increase in temperature.
Learn more about Le Chatelier's principle
brainly.com/question/29009512
#SPJ11
Question 9What pressure will be exerted by 15 g of carbon dioxide gas in a 20 L container at 0 °C?
Answers
In this question, we need to find the value of pressure of a 15 grams sample of CO2 gas, and in order to find this value, we will be using the Ideal gas law formula, which is the following:
PV = nRT
Where:
P = pressure in atm
V = volume in liters, 20 Liters
n = number of moles
R = gas constant, 0.082
T = temperature in kelvin, 0°C = 273 K
The number of moles we need to find based on the mass in the question and based on the molar mass of CO2, 44g/mol:
44g = 1 mol
15g = x moles
44x = 15
x = 15/44
x = 0.34 moles of CO2 in 15 grams
Now we have the values to add to the formula:
P * 20 = 0.34 * 0.082 * 273
20P = 7.61
P = 7.61/20
P = 0.38 atm of pressure
The pressure will be 0.38 atm
Use the picture below to answer the question. Comparing Wave A (blue) to Wave B (red), Wave B has a

Answers
Answer: B longer wave
Explanation:
because
Someone help me with this pls

Answers
Answer:
D
Explanation:
Lamps
when the n quantum number equals 1 we are in what orbital
A)d
B) f
C)p
D)s
Answers
i think it is D i hope this helps yall
A 46 g sample of metal absorbs 250 J and the temperature changes from 25.0°C to 31 0°C. What is the specific heat of this unknown metal?
Answers
q = m * c * ΔT
where q is the heat energy absorbed by the metal, m is the mass of the metal, c is the specific heat of the metal, and ΔT is the change in temperature of the metal.
Substituting the given values, we get:
250 J = 46 g * c * (31.0°C - 25.0°C)
Simplifying the equation, we get:
250 J = 46 g * c * 6.0°C
Dividing both sides by (46 g * 6.0°C), we get:
c = 250 J / (46 g * 6.0°C)
c = 0.906 J/(g°C)
Therefore, the specific heat of the unknown metal is 0.906 J/(g°C).
is this a good college to go

Answers
Two intermetallic compounds, A3B and AB3, ex- ist for elements A and B. If the compositions for A3B and AB3 are 91.0 wt% A–9.0 wt% B and 53.0 wt% A–47.0 wt% B, respectively, and element A is zirconium, identify element B.
Answers
Element B has an atomic weight of 26.67 g/mol, and element A has an atomic weight of 91.22 g/mol, which means that element B is niobium (Nb), whose atomic weight is 92.91 g/mol.
To solve this problem, we need to use the information given about the compositions of the intermetallic compounds A3B and AB3 and the identity of element A, which is zirconium (Zr).
Let x be the atomic weight of element B.
In A3B, the composition is 91.0 wt% A and 9.0 wt% B. This means that the atomic weight of A is 91.0/atomic weight of A3B and the atomic weight of B is 9.0/atomic weight of A3B. Using the atomic weight of zirconium, which is 91.22 g/mol, we can set up the following equation:
91.0/91.22 + 9.0/x = 3/1
Simplifying this equation, we get:
0.9974 + 9.0/x = 3
Multiplying both sides by x, we get:
0.9974x + 9.0 = 3x
Solving for x, we get:
x = 26.67 g/mol
Therefore, element B has an atomic weight of 26.67 g/mol.
In AB3, the composition is 53.0 wt% A and 47.0 wt% B. Using the atomic weight of zirconium and the atomic weight of element B, we can calculate the atomic weight of A as follows:
53.0/91.22 + 47.0/x = 1/3
Simplifying this equation, we get:
0.5817 + 47.0/x = 0.3333
Multiplying both sides by x, we get:
0.5817x + 47.0 = 0.3333x
Solving for x, we get:
x = 121.08 g/mol
Therefore, element B has an atomic weight of 26.67 g/mol, and element A has an atomic weight of 91.22 g/mol, which means that element B is niobium (Nb), whose atomic weight is 92.91 g/mol and is closest to our calculated atomic weight of 26.67 g/mol.
For more such questions on Atomic weight
https://brainly.com/question/13399225
#SPJ11
Which of the following statements about the molecular orbital (MO) theory is true?
a. When two p orbitals of similar phase overlap side-by-side, a π
∗
antibonding molecular orbital is formed.
b. When two p orbitals of opposite phase overlap side-by-side, a π
bonding molecular orbital is formed.
c. A π
bonding molecular orbital is higher in energy than the two atomic p orbitals from which it is formed.
d. A π
∗
bonding molecular orbital is higher in energy than the two atomic p orbitals from which it is formed.
Answers
The correct statement about molecular orbital (MO) theory is: d. A π∗ (pi star) antibonding molecular orbital is higher in energy than the two atomic p orbitals from which it is formed.
In MO theory, when two atomic orbitals combine, they form molecular orbitals. There are two types of molecular orbitals: bonding and antibonding. Bonding molecular orbitals result from the constructive interference of atomic orbitals, leading to lower energy and increased stability. Antibonding molecular orbitals, on the other hand, form due to the destructive interference of atomic orbitals, resulting in higher energy and decreased stability.
For statement d, when two p orbitals of similar phase overlap side-by-side, a π bonding molecular orbital is formed, not an antibonding molecular orbital as stated in option a. Statement b is incorrect because a π bonding molecular orbital forms when two p orbitals of similar phase overlap side-by-side, not of opposite phase. Finally, statement c is incorrect because a π bonding molecular orbital is lower in energy, not higher, than the two atomic p orbitals from which it is formed.
Learn more about molecular orbital (MO) theory here:-
https://brainly.com/question/30028700
#SPJ11
Heat packs are used to relax muscles. Some heat packs contain iron in a small packet. To
activate this type of heat pack, you need to increase the pressure and introduce oxygen
to create iron(III) oxide which produces the heat needed for muscles to relax. This
reaction in an example of what type of reaction?
Answers
Heat packs are used to relax muscles. This reaction is an example of exothermic reaction.
What is exothermic reaction?Exothermic reaction is defined as a reaction that is chemical in nature and is characterized by the release of energy in the form of heat and light.
It can also be defined as a thermodynamic process or reaction that releases energy from system to its surroundings.
Endothermic reaction is defined as the chemical reaction in which the reactants absorbs heat energy from the surroundings to form product.
Endothermic reaction can also be defined as a reaction in which the system absorbs energy from its surroundings in the form of heat.
Being endothermic enables us to live in cooler environments and regulate our body temperatures in order to fight infection.
Thus, heat packs are used to relax muscles. This reaction is an example of exothermic reaction.
To learn more about exothermic reaction, refer to the link below:
https://brainly.com/question/10373907
#SPJ5
SOMEONE HELP ME PLEASE
Convert the answer above to Kelvin

Answers
Answer:
313.15
Explanation:
Green plants use light from the Sun to drive photosynthesis, a chemical reaction in which liquid water and carbon dioxide gas form aqueous glucose C6H12O6
and oxygen O2
gas. Calculate the moles of water needed to produce 1.00mol
of glucose. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Answers
Answer:
Explanation:
The photosynthetic equation is as follows:
6CO2 + 6H2O = C6H12O6 + 6O2
According to this equation, 6 moles of water (H2O) is required to produce 1 mole of glucose
Which of the following is a stable ion that exists under ordinary conditions?
N3-
Al+
Li2+
F2-
Answers
Answer:
Hmm 65
Explanation:
chemically balance the equation: Mg(HCO3)2 + HCL --> MgCl2 + CO2 + H2O
Answers
Answer:
Mg(HCO3)2 + 2HCl → 2CO2 + 2H2O + MgCl2
Explanation:
Mg(HCO3)2 + 2HCl → 2CO2 + 2H2O + MgCl2
Which condition is sufficient to show ABC~QPR?
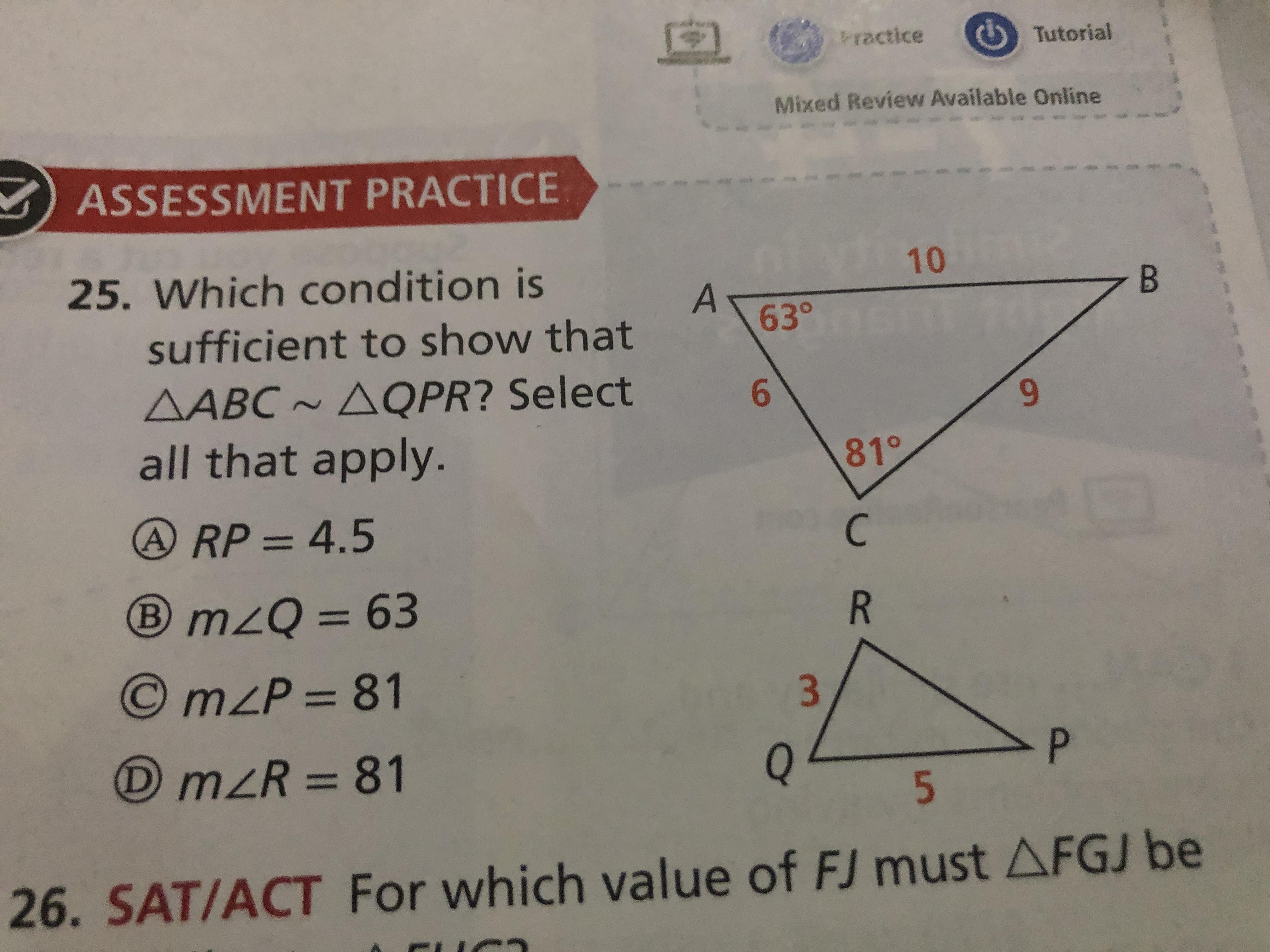
Answers
For AABC AQPR to be true, the measures of angles A, C, and D must all be equal; in this case, they must all be equal to 81°. Therefore, conditions A, C, and D are sufficient to show that AABC AQPR.
What is angles ?Angles are points in a plane that are formed when two lines intersect. They can also be formed when one line rotates around a fixed point. Angles are measured in degrees and can be equal to, greater than, or less than a right angle (90°). The four main types of angles are acute (less than 90°), right (90°), obtuse (greater than 90°), and straight (180°). Angles can also be classified as complementary, supplementary, vertical, alternate, and adjacent.
To learn more about angles
https://brainly.com/question/25425872
#SPJ1
Can someone plz help me? :(

Answers
Answer:
it's C
Explanation:
because it exhaled the carbon dioxide
hich of the following is true concerning ideal gases? responses a gas exerts pressure as a result of collisions of the gas molecules with the walls of the container. a gas exerts pressure as a result of collisions of the gas molecules with the walls of the container. the gas particles in a sample exert attraction for one another. the gas particles in a sample exert attraction for one another. at stp, 1.0 l of ar (g) contains about twice the number of atoms as 1.0 l of ne (g) since the molar mass of ar is about twice that of ne. at stp, 1.0 l of ar (g) contains about twice the number of atoms as 1.0 l of ne (g) since the molar mass of ar is about twice that of ne. the temperature of the gas sample is directly related to the average velocity of the gas particles.
Answers
A gas exerts pressure as a result of collisions of the gas molecules with the walls of the container.
What is an Ideal gas?
An ideal gas is a hypothetical gas that perfectly complies with the gas laws since its molecules take up very little space and don't interact
No attraction or repellence exists between the molecules of ideal gases. The sole interaction between molecules of an ideal gas would be an elastic collision when they collided or an elastic collision with the container walls.
The molecules of an ideal gas have no volume. The molecules of the gas take up the volume because they spread out over a broad area of space, whereas the molecules of an ideal gas are represented as point particles with no volume on their own.
For more questions like Ideal gas click the link below:
https://brainly.com/question/11607022
#SPJ4

Which elements will form a cation?
Oxygen
Helium
Magnesium
Sulfur
Answers
Answer:
Magnesium
Explanation:
Magnesium is a metalMetals have tendency to give up the electrons of the outermost shell and forms cation.\(Mg \longrightarrow Mg^{2+} + 2e^-\)What volume of water can be boiled by 3.0 kJ of energy? (Refer to table of
constants for water.)
A. 3.0 kJ x
1 mol
x 18.02 g/mol
1 mL
= 13 mL
1 g
4.186 kJ
B. 3.0 kJ x 1 mol
* 18.02 g/mol
6.03 kJ
1 mL
= 9.0 mL
1 g
C. 3.0 kJ x
1 mol
x 18.02 g/mol
(-285.83 kJ)
1 mL
1g
= 0.19 mL
1 mL
D. 3.0 kJ x
1 mol
40.65 kJ
* 18.02 g/mol
= 1.3 mL
SUBMIT
Answers
Answer: 3.0 kJ × 1 mol/40.65 kJ× 18.02 g/mol × 1 mL/1 g= 1.3 mL
Consider the Baeyer permanganate test and chromic acid tests. These tests work by converting an aldehyde to what functional group? 1 KMnO4 and H2CrO4 act as what kind of reagent? (e.g. electrophile, nucleophile, oxidizing agent, reducing agent, acid catalyst, base catalyst, solvent etc.) 2. 3. Why does a ketone not react with these reagents?
Answers
The Baeyer permanganate test and chromic acid tests work by converting an aldehyde to a carboxylic acid functional group.
KMnO₄ and H₂CrO₄ act as oxidizing agents. A ketone does not react with these reagents because it does not have a hydrogen atom attached to the carbonyl group.
How does the Baeyer permanganate test work?The Baeyer permanganate test is used to identify the presence of unsaturation (i.e. double bonds) in a compound. When a double bond is present in the compound, it will be oxidized by potassium permanganate (KMnO₄) to form a diol functional group. In the case of aldehydes, the double bond is present between the carbonyl carbon and the hydrogen atom.
Therefore, the reaction will convert an aldehyde to a carboxylic acid functional group. This reaction is also known as the oxidation of aldehydes with KMnO₄.
What is the chromic acid test?The chromic acid test is another method for identifying the presence of unsaturation in a compound. It uses chromic acid (H₂CrO₄) as the oxidizing agent. Like the Baeyer permanganate test, the chromic acid test will convert an aldehyde to a carboxylic acid functional group. It is important to note that the chromic acid test is more sensitive to the presence of double bonds than the Baeyer permanganate test.
Therefore, it is often used as a confirmatory test after a positive result is obtained from the Baeyer permanganate test.
Learn more about chromic acid test on:
https://brainly.com/question/30824151
#SPJ11
Alyssa repeated the titration of a 5.00 mL antimony trichloride solution with distilled water until a slightly cloudy appearance persisted after thoroughly mixing the solution. Based on her data, she calculated the following concentrations for SbCl3 and HCl. Calculate the equilibrium constant, K, for the hydrolysis of the antimony trichloride.
Concentration of SbCl3 = 0.046 M
Concentration of HCl = 2.1 M
PART B.
Consider the following equilibrium, for which Kc = 448 at 23 ˚C
N2 (g) + O2 (g) + Br2 (g)\rightleftharpoons2 NOBr (g):
What is the value of Kp for this reaction?
Answers
The value of Kp for the given reaction is 12.2 atm. We can find it in the following manner.
PART A:
The hydrolysis reaction of antimony trichloride can be written as follows:
SbCl₃ + 3H₂O ⇌ Sb(OH)₃ + 3HCl
The equilibrium constant expression for this reaction can be written as:
K = [Sb(OH)₃][HCl]³ / [SbCl₃][H₂O]₃
The concentration of SbCl₃ is given as 0.046 M, and the concentration of HCl is given as 2.1 M. Assuming the volume of water used for dilution is negligible, the concentration of H2O can be considered to be 55.5 M (at 25 ˚C). The concentration of Sb(OH)₃ can be calculated using the stoichiometry of the reaction:
0.046 M SbCl3 x (1 mol Sb(OH)3 / 1 mol SbCl₃) = 0.046 M Sb(OH)₃
Substituting the given values into the equilibrium constant expression, we get:
K = (0.046 M) x (2.1 M)³ / (1)³x (55.5 M)³
K = 1.7 x 10⁻¹⁰
Therefore, the equilibrium constant, K, for the hydrolysis of antimony trichloride is 1.7 x 10⁻¹⁰.
PART B:
To calculate Kp for the given reaction, we can use the relationship between Kc and Kp, which is:
Kp = Kc(RT)^Δn
where R is the gas constant (0.0821 L atm mol⁻¹ K⁻¹), T is the temperature in Kelvin, and Δn is the difference between the total number of moles of gaseous products and the total number of moles of gaseous reactants.
In this case, Δn = (2 - 1 - 1) = 0, since the total number of moles of gaseous products (2 moles of NOBr) is equal to the total number of moles of gaseous reactants (1 mole of N2, 1 mole of O2, and 1 mole of Br2).
Substituting the given values into the equation for Kp, we get:
Kp = (448)(0.0821 L atm mol^-1 K⁻¹)(296 K)⁰
Kp = 12.2 atm
Therefore, the value of Kp for the given reaction is 12.2 atm.
Learn more about hydrolysis here brainly.com/question/29439050
#SPJ4
Tungsten fits best into which category?
A. molecule
B. element
C. compound
D. mixture
Answers
Tungsten is an element. It is located in the d -block of periodic table. Thus it is a transition metal. Hence, option B is correct.
What is tungsten ?Tungsten is 74th element in periodic table. It is a transition metal thus, located in the d-block of periodic table. Tungsten has the chemical symbol W. Tungsten was first identified as an element in 1781.
The maximum melting temperature of any known element other than carbon, 3,422 °C, is reached by the unbound element, making its resistance all the more remarkable (which sublimes at atmospheric pressure). Tungsten has a boiling point of around 5930°C.
A few of the many alloys that contain tungsten and have many applications include radiation shielding, X-ray tubes, electrodes in gas tungsten arc welding, superalloys, and incandescent light bulb filaments.
Find more on tungsten :
https://brainly.com/question/1455426
#SPJ6
question
question
e
What volume (in L) will a 32 g sample of butane gas, C4H10(9), occupy at a temperature of 45.0 °C and a pressure of 728
mm Hg?
Answers
Answer:
The volume of the gas is 0.015 m^3.
Explanation:
mass, m = 32 g
Temperature, T = 45 °C = 45 + 273 = 318 K
Pressure, P = 728 mm of hg = 0.728 x 13.6 x 1000 x 9.8 = 97027.84 Pa
Atomic mass = 4 x 12 + 10 x 1 = 58 g
Use the ideal gas equation
Let the volume is V.
P V = n R T
\(97027.8 \times V = \frac{32}{58}\times 8.31 \times 318 \\\\V = 0.015 m^3\)
Explanation:
The volume of the gas occupied can be calculated by using the ideal gas equation:
\(PV=nRT\)
where,
P=pressure of the gas in atm
V=volume of the gas in L.
n=number of moles of the gas
R=0.0821L.atm.mol-1.K-1
T=absolute temperature
To get the volume of the gas, follow the below steps:
1) Calculate the number of moles of gas:
Number of moles of butane=mass of butane given/its molecular mass
\(=32g/58.0g/mol\\=0.55mol\)
2) Convert temperature into kelvin scale:
T=(45+273)K=318K
3)Convert pressure into atm:
760 mm Hg =1 atm
then,
728 mm Hg=
728 mm Hg x 1 atm /760 mm Hg
=0.957 atm
Substitute all these values in the ideal gas equation to get the volume:
\(V=\frac{nRT}{P} \\V=0.55mol x 0.0821 L.atm.mol-1.K-1 x 318K / 0.957 atm\\V=15.0L\)
Answer:
The volume of butane gas is 15.0 L
The boiling point of SeOF2 is less than the boiling point of SeOCl2.
a) Identify the type(s) of intermolecular force(s) that the two substances have in common.
b) Explain the difference in boiling points based on the types and relative strengths of intermolecular forces.
Answers
The intermolecular force that is common to both is the dipole forces
b) The lesser molecular mass of \(SeOF_{2}\) made it to have a lesser boiling point.
What is the intermolecular forces?We know that apart from the forces that holds the atoms together in a molecule, there is yet another force that holds the molecules together in a particular state of matter. Let us recall that matter is anything that has mass and occupy space.
If we look at the two compounds that we have, it is clear that the commonality between the two compounds is that the both of them are held together by the dipole forces because the molecules are composed of atoms that are polar.
On the other hand, there is a dispersion force that is found in every molecule. The magnitude of the dispersion forces depends on the molecular mas of the molecule. Given that \(SeOCl_{2}\) has a greater molecular mass than \(SeOF_{2}\), it would necessarily have grater boiling point than the later.
Learn more about intermolecular forces:https://brainly.com/question/9007693
#SPJ1
Is farting an example of an exothermic reaction?
*for real though I have never heard of a cold fart
Answers
Answer:
i would assume i mean who has cold farts
Explanation:
Yes, farting is an example of an exothermic reaction.
What is an exothermic reaction?A reaction that is chemical in nature and is characterized by the release of energy in the form of heat or light is called an exothermic reaction.
Farting is a normal part of digestion that reflects the activity of the bacteria in your gut.
Yes, farting is an example of an exothermic reaction.
Learn more about the exothermic reaction here:
https://brainly.com/question/10373907
#SPJ2
Which element is most likely to react with Br?
- Fe
- N
- Ar
- Li
Which element is least likely to react with Br?
- Fe
- N
- Ar
- Li
Answers
The element is most likely to react with Br is Li(Lithium). Alkali metals have very low density, which makes them very reactive, because they want to gain energy and become stable.
Lithium is an alkali metal which belongs to group 1 . Bromine (Br) reacts with many metals, sometimes very vigorously. For instance, with potassium, it reacts explosively. Bromine even combines with relatively unreactive metals, such as platinum and palladium. The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. Lithium, sodium, and potassium all react with water, for example.
Out of the given elements the correct choice is Li.
To learn more about alkali metals check the link below-
https://brainly.com/question/19109836
#SPJ4
An enzyme serves as a place where a chemical reaction can occur because? a. the enzyme is a substrate.
b. the enzyme is one of the reactants in the reaction.
c. the charge on the enzyme's active site is the same as the charge on the substrates.
d. the shape of the substrates fits perfectly into the shape of the enzyme's active site.
Answers
An enzyme serves as a place where a chemical reaction can occur because the correct option is d. the shape of the substrates fits perfectly into the shape of the enzyme's active site.
The Enzymes are the proteins that help to speed up the chemical reactions in our bodies. The Enzymes are the essential for the digestion, the liver function and much more. The substrate is the substance on which the enzyme operates in an enzymatic reaction.
Thus, the shape of the substrates will fits perfectly into the shape of the enzyme's active site, this is the reason enzyme serves as the place where the chemical reaction can occur.
To learn more about enzyme here
https://brainly.com/question/11816222
#SPJ4
How does the ionosphere affect radio frequencies?
Group of answer choices
FM frequencies are not reflected and shortwave frequencies are bounced off the ionosphere and back to Earth several times.
FM frequencies are bounced back to Earth and back several times.
Shortwave radio frequencies are absorbed by the ionosphere and FM frequencies pass through.
Shortwave radio frequencies are not affected and pass through the ionosphere.
Answers
Answer:Shortwave radio frequencies are not affected and pass through the ionosphere.
Explanation:
for the following equilibrium, mn(oh)2(s)↽−−⇀mn2 (aq) 2oh−(aq) what adjustment would result in further precipitation?
Answers
The following adjustment would result in further precipitation of the given equilibrium: Decreasing the concentration of OH
The given equilibrium reaction is shown below:mn(oh)2(s)↽−−⇀mn2 (aq) 2oh−(aq)According to the Le Chatelier’s principle, if a stress is applied to a system at equilibrium, the equilibrium will shift in a direction that reduces the stress. So, in order to shift the equilibrium in the forward direction, stress must be applied to the reverse reaction.
This can be done by decreasing the concentration of OH- ions which will result in the precipitation of more Mn(OH)2. Hence, decreasing the concentration of OH- will result in further precipitation of the given equilibrium.In other words, when the concentration of hydroxide ions is reduced, the Mn2+ and OH- ions will react with each other in the forward direction to compensate for the loss of hydroxide ions.
To know more about concentration visit:
https://brainly.com/question/3045247
#SPJ11
?A scientist is investigating how the lunar cycle affects the number of sea turtles nesting each night. Which of the following is the dependent variable?
Answers
Answer:
Number of nesting turtles
Explanation:
The dependent variable is the Number of nesting turtles.
How does the Moon affect sea turtles?
The lunar phase may also affect leatherback nesting visually. On clear nights when the Moon is full, visibility may be greater and the presence of tourists and egg predators may discourage turtles from emerging.
Why do sea turtles follow the Moon?
When making their way back to the ocean, sea turtles use the light of the Moon and stars to navigate. Sea turtles use the light of the Moon and stars to navigate. Artificial lighting from street lights, buildings, and flashlights on the beach can disrupt their ability to find their way back to the water
Learn more about Sea Turtles at https://brainly.com/question/27625739
#SPJ2