How many Elements are in the compound C2H2O ?
A. 3
B. 2
C. 0
D. 1
Answers
Answer:
3 (carbon, hydrogen, oxygen)
Related Questions
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
7. What is one of the BEST actions the US government can take to slow global warming?
-provide tax credits to those who carpool
-encourage people to buy electronic books versus paperbacks or hardbacks
-produce commercials that urge people to recycle
-invest in clean technologies such as wind and solar power
Answers
one of the BEST actions the US government can take to slow global warming is invest in clean technologies such as wind and solar power
What is the US government doing about global warming?EPA works with industry and others to reduce greenhouse gas emissions through regulatory initiatives and partnership programs. Within the Agency, EPA implements a range of strategies to reduce its own greenhouse gas emissions, increase energy efficiency, and take other steps to reduce its carbon footprintReaching 100% carbon pollution-free electricity by 2035. Achieving a net-zero emissions economy by 2050. Delivering 40% of the benefits from federal investments in climate and clean energy to disadvantaged communitiesMobilizing a whole-of-government approach, the United States is scaling up action at home and abroad to put the world on a path to reach net-zero emissions by 2050 and to achieve the global goal on adaptation. Learn more about the United States at COP27 and the U.S. Center
To learn more about global warming refers to:
brainly.com/question/26402403
#SPJ1
100 POINTS WILL MARK BRAINLIEST PICTURE BELOW

Answers
Answer:
D
Explanation:
it seems the most logical
Answer:
B
Explanation:
6.00 ml
PLEASE MARK AS BRAINLIEST
what is e°cell for the following reaction? 2ag(s) sn2 (aq) → 2ag (aq) sn(s) ag (aq) e– → ag(s) e° = 0.80 v sn4 (aq) 2e– → sn2 (aq) e° = 0.13 v sn2 (aq) 2e– → sn(s) e° = –0.14 v
Answers
The standard cell potential of the reaction is 0.67 V obtained by subtracting the reduction and oxidation half-reaction potentials.
How to find standard cell potential?To find the standard cell potential, we can use the formula:
E°cell = E°(reduction at cathode) - E°(oxidation at anode)
First, let's write the overall balanced equation for the reaction:
2Ag(s) + Sn₄+(aq) → 2Ag+(aq) + Sn₂+(aq)
The reduction half-reaction occurs at the cathode, where Ag+ ions are reduced to Ag(s):
Ag+(aq) + e- → Ag(s) E° = 0.80 V
The oxidation half-reaction occurs at the anode, where Sn₄+ ions are oxidized to Sn₂+ ions:
Sn₄+(aq) + 2e- → Sn₂+(aq) E° = 0.13 V
Notice that the reduction half-reaction has a higher E° value than the oxidation half-reaction, which means it is more likely to occur spontaneously. To get the overall cell potential, we subtract the oxidation half-reaction potential from the reduction half-reaction potential:
E°cell = E°(reduction at cathode) - E°(oxidation at anode)
E°cell = 0.80 V - 0.13 V
E°cell = 0.67 V
Therefore, the standard cell potential for the given reaction is 0.67 V.
Learn more about standard cell
brainly.com/question/28188023
#SPJ11
Sulfur is a non metallic material that has a density of 2g/cm3. The volume of a sample of sulfur was measured to be 5.0cm3. What is the density of the wood?
Answers
question: Sulfur is a non metallic material that has a density of 2g/cm3. The volume of a sample of sulfur was measured to be 5.0cm3. What is the mass of the sulfur
Answer:
0.01 kg
Explanation:
Density: This can be defined as the ratio of mass of a body and it's volume. The S.I unit of volume is kg/m³.
Therefore,
Density = mass/volume
D = m/V......................................................... Equation 1
making m the subject of the equation
m = D×V ................................... Equation 2
Given: D = 2 g/cm³ = 2000 kg/m³, V = 5.0 cm³ = 0.000005 m³
subtitute into equation 2
m = 2000×0.000005
m = 0.01 kg or 10 g
are bath bombs covalent or ionic bonds?
Answers
What is the average atomic mass of
the element in the data table?
Mass (amu)
38.96
39.96
40.96
Abundance (%)
93.26
0.01
6.73
[?]amu
![What is the average atomic mass ofthe element in the data table?Mass (amu)38.9639.9640.96Abundance (%)93.260.016.73[?]amu](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/ZnSSS1JEgfg1fIpVlq9U62m71gNXuvvo.png)
Answers
Answer:
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance.
on what bases are the elements of periodic table classified?
Answers
The elements can also be classified into the main-group elements (or representative elements) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12; and inner transition metals in the two rows at the bottom of the table (the top-row elements are called lanthanides and the bottom-row ...
Hello,
What are Elements?Elements are a group of the Periodic Table that shares common valence electron structures. They are arranged in order of increasing Atomic Number.
Now back to the question:-In modern periodic table they are classified into three parts- metals, non-metals and metalloids.
In detail:-
• Metals- Metals are those elements which donate electrons. Ex- Na, K, Rb, Mg, Ca, etc.
• Non-metals- Non-metals are those elements which accept electrons. Ex- N, O, F, Cl, etc .
• Metalloids- Metalloids are those elements which show property of both metals and non-metals. Ex- B, Si, Ge, etc.
Hope it helps...
(by Benjemin)
Which of the following elements has the same number of valence electrons as aluminum?
zinc
carbon
silicon
magnesium
or boron
Answers
Answer:
boron
Explanation:
Boron and aluminum lie in same group in modern periodic table and both have three valence electrons
what is the mass of 0.75 mol of hydrogen sulfide
Answers
Answer:
25.56066
Explanation:
hope this helps !!!!!!! and sorry if it doesn't help
help me with this plssss


Answers
Answer:
5=D
6=B
7=A
8=?????
1A= conduction
1B= radiation
1C= convection
3: C
4: A
Explanation:
2: The heat from the hot water is been transferred along the metal handle to the other end of the spoon by the process of CONDUCTION.
How many moles of NH3 can you make from 6.20 moles of N2?
Answers
$ hope it welp
The term mole concept is used here to determine the moles of ammonia. The number of moles of ammonia which can be make from 6.20 moles of N₂ is 12.4.
What is a mole?One mole of a substance is defined as that quantity of it which contains as many entities as there are atoms exactly in 12 g of carbon - 12. The formula used to calculate the number of moles is:
Number of moles = Given mass / Molar mass
You need one nitrogen atom to produce ammonia. Here we can see that there are two nitrogen atoms in N₂.
One mole of any substance contains Avogadro number of molecules. A mole is defined as the mass of the substance which consists of the equal quantity of basic units.
The number of moles of ammonia from 6.20 moles of N₂ is:
6.20 × 2 = 12.4
Thus the number of moles is 12.4.
To know more about mole concept, visit;
https://brainly.com/question/19730733
#SPJ2
what is the numerical ratio of the rate of change in the concentration of co2 to the rate of change in the concentration of o2
Answers
The numerical ratio of the rate of change in the concentration of CO2 to the rate of change in the concentration of O2 depends on the specific conditions and context being considered.
Numerical ratio explained.
The numerical ratio of the rate of change in the concentration of CO2 to the rate of change in the concentration of O2 depends on the specific conditions and context being considered. However, in general, the ratio of the rate of change in the concentration of CO2 to the rate of change in the concentration of O2 can provide insights into various biological, ecological, and atmospheric processes.
For example, in the process of cellular respiration, the ratio of CO2 produced to O2 consumed is 1:1, meaning that the rate of change in the concentration of CO2 is equal to the rate of change in the concentration of O2.
In contrast, during photosynthesis, plants and other autotrophs take in CO2 and release O2. The ratio of the rate of change in the concentration of CO2 to the rate of change in the concentration of O2 during photosynthesis depends on various factors such as the type of plant, the amount of light and nutrients available, and the surrounding temperature and humidity.
Therefore, the numerical ratio of the rate of change in the concentration of CO2 to the rate of change in the concentration of O2 varies depending on the specific context and conditions being considered.
Learn more about numerical ratio below.
https://brainly.com/question/29145263
#SPJ1
What type of reaction is this?
N2 + 3H2 → 2NH3
Answers
5) To check the accuracy of our results we will compare our results to the label on the vinegar bottle. The bottle contains 4% vinegar. We will need to change our M results to %% in order to calculate a percent error.
Using the average M and the average volume (you have to change it to LITERS) of the acetic acid find the # of moles of acetic acid using the molarity formula from Table T.
Change moles to grams using the gfm of acetic acid (HC,H,O,).
Divide grams of acetic acid by the average volume (this time in ml.) of acetic acid and then multiply by 100. This is your experimental %.
Calculate the % error.
6. What other indicator could we have used?
7. What adjustment to our calculations would we have needed to make if we used barium hydroxide rather than sodium hydroxide? (It might be helpful to write the formula for barium hydroxide
Answers
5) Convert molarity to percent, calculate moles of acetic acid, convert moles to grams, divide grams by volume in mL, multiply by 100 to obtain experimental percent, and calculate percent error.
6) Phenolphthalein could have been used as an alternative indicator.
7) When using barium hydroxide instead of sodium hydroxide, adjust the calculations by considering the stoichiometry of the reaction and using a molar ratio of 2:1 between acetic acid and barium hydroxide.
5. To calculate the percent error in the concentration of acetic acid, we need to convert our molarity (M) results to percent (%). Using the average molarity and the average volume (converted to liters) of acetic acid, we can calculate the number of moles of acetic acid.
Then, by converting moles to grams using the molar mass of acetic acid (CH3COOH), we can divide the grams of acetic acid by the average volume (in milliliters) of acetic acid and multiply by 100 to obtain the experimental percent.
Finally, we can calculate the percent error by comparing the experimental percent to the labeled percent (4% vinegar on the bottle).
6. An alternative indicator that could have been used is phenolphthalein. Phenolphthalein is commonly used in acid-base titrations and changes color in a specific pH range, indicating the endpoint of the reaction.
6. If barium hydroxide (Ba(OH)2) were used instead of sodium hydroxide (NaOH), the adjustment in calculations would involve the stoichiometry of the reaction. The balanced chemical equation for the reaction between acetic acid and barium hydroxide is:
2CH3COOH + Ba(OH)2 → Ba(CH3COO)2 + 2H2O
The molar ratio between acetic acid and barium hydroxide is 2:1. Therefore, the number of moles of barium hydroxide used would be half the number of moles of acetic acid in the calculation.
The rest of the procedure, including converting moles to grams and calculating the percent, would remain the same.
For more such questions on molarity visit:
https://brainly.com/question/30404105
#SPJ8
Which of the following is not true for thermoplastic polymers?
A) Thermoplastics are linear polymers.
B) They often melt on heating.
C) Molten polymer can be remolded into any shape.
D) They have cross-linkage which breaks on heating.
Answers
The statement that is not true for thermoplastic polymers is D) They have cross-linkage which breaks on heating.
Thermoplastic polymers are characterized by their linear molecular structure (A), which allows them to be melted and reshaped multiple times without significant degradation in properties. This is because their molecular chains can slide past one another when heated, leading to a softened or molten state (B). Once in this state, the polymer can be easily molded or extruded into various shapes (C) and will solidify upon cooling.
In contrast, thermosetting polymers exhibit cross-linking between their molecular chains, forming a three-dimensional network that provides strength and stability. This cross-linking prevents them from being remolded upon heating. Instead, thermosetting polymers undergo a curing process, where the cross-links are formed through heat or chemical reactions, rendering the material in a permanent, rigid state. Therefore, statement D is incorrect as it describes a characteristic of thermosetting polymers, not thermoplastics.
Learn more about Thermoplastic polymers here: https://brainly.com/question/29853071
#SPJ11
Use the chemical equation to answer the question.
WO3(s) + 3H2(g) → W(s) + 3H2O(g)
Which statement correctly describes the reaction?
(1 point)
Tungsten (W) changes oxidation numbers from +6 to zero, so it undergoes oxidation.
Oxygen (O) changes oxidation numbers from –2 to zero, so it undergoes reduction.
Tungsten (W) changes oxidation numbers from +6 to zero, so it undergoes reduction.
Oxygen (O) changes oxidation numbers from –2 to zero, so it undergoes oxidation
Answers
The statement that correctly describes the reaction is: Tungsten (W) changes oxidation numbers from +6 to zero, so it undergoes reduction.
WHAT IS OXIDATION AND REDUCTION:Oxidation is the process whereby electrons are lost in a reaction while reduction involves the gain of electrons.According to this question, the following reaction is given: WO3(s) + 3H2(g) → W(s) + 3H2O(g)Tungsten has an oxidation number of +6 in WO3 and it is changed to 0 in W, hence, there is a gain of electrons by tungsten (W).Therefore, the statement that correctly describes the reaction is: Tungsten (W) changes oxidation numbers from +6 to zero, so it undergoes reduction.
Learn more about oxidation-reduction reaction at: https://brainly.com/question/3867774
Combustion analysis of a hydrocarbon produced
0.40g of CO2 and 0.205g of H20. Calculate the
empirical formula of the hydrocarbon.
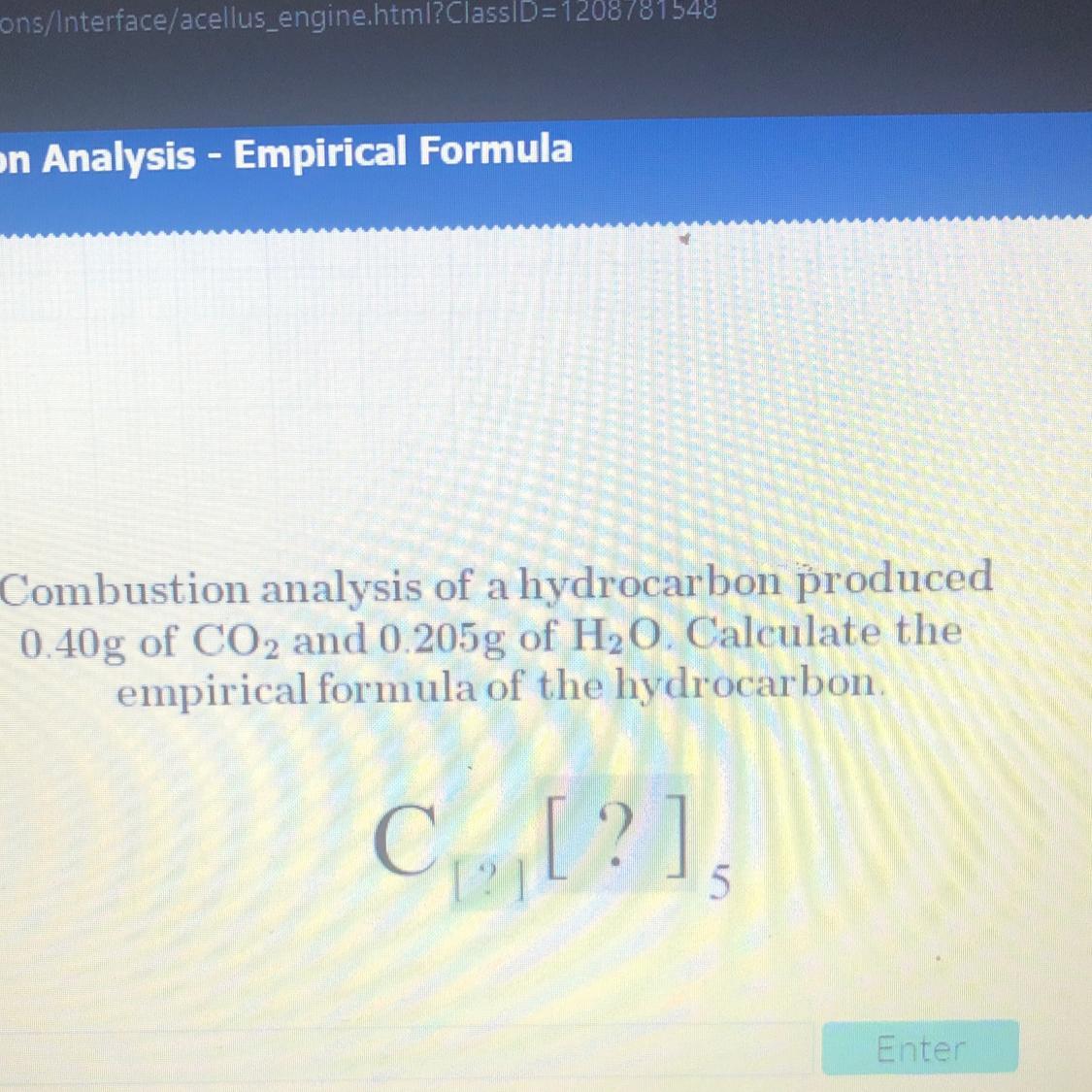
Answers
Do the same for H2O except the mole ratio is 2 mol H / 1 mol H2O, since every mole of H2O has two moles of hydrogen in it.
0.40 g CO2 • 1 mol CO2/44.01 g CO2 • 1 mol C / 1 mol CO2 = 0.0091 mol C
0.205 g H2O • 1 mol H2O/18.02 g H2O • 2 mol H / 1 mol H2O = 0.0228 mol H
Now divide by the smallest number of moles (0.0091 moles of C) to get whole number values for both C and H rather than decimals.
0.0091/0.0091 = 1 mol C
0.0228/0.0091 = 2.5 mol H
You need whole numbers for both C and H, which you can get by just multiplying both by 2 (since 2.5 • 2 is 5).
So, your empirical formula ends up being C2H5.
1. Give the best advice on being understanding but kinda flirty to a friend that is a girl.
2. You can add a pickup line. :)
THIS IS AN EMERGENCY.
Answers
give the correct charge for ions of the following elements. drag the appropriate labels to their respective targets. note: not all labels will be used. resethelp request answer provide feedback
1+ 3+ Ca
2+ Cl
0 O
1- Al
2- K
Answers
The correct charge for ions of the given elements are as follows: Calcium (Ca) has a 2+ charge. Chlorine (Cl) has a 1- charge. Oxygen (O) has a 2- charge. Aluminium (Al) has a 3+ charge.
The symbols for the other ions are not provided, so we cannot determine their charge. I hope this answer helps.
For more similar questions on topic Chlorine
https://brainly.com/question/31274929
#SPJ11
Which of the following are conjugate acid/base pairs? Select all that apply. H2PO 4−and HPO4^2−H2CO3 and CO 3^2−
HCl and NaOH H3O + and OH − HCl and Cl−
Answers
A conjugate acid-base pair is the pair of two compounds which differ by the presence of a proton. An acid will donate protons to a base and become a conjugate base. A base will accept protons and become a conjugate acid.Conjugate acid/base pairs are as follows:H2PO4− and HPO42−H2CO3 and CO32−HCl and Cl−H3O+ and OH−Explanation:
H2PO4− and HPO42−H2PO4− can donate a proton to become HPO42− and the latter can accept a proton to become H2PO4−.H2CO3 and CO32−H2CO3 can donate a proton to become CO32− and the latter can accept a proton to become H2CO3.HCl and Cl−HCl can donate a proton to become Cl− and the latter can accept a proton to become HCl.H3O+ and OH−H3O+ can donate a proton to become OH− and the latter can accept a proton to become H3O+.Therefore, the following are conjugate acid/base pairs:H2PO4− and HPO42−H2CO3 and CO32−HCl and Cl−H3O+ and OH−
to know more about conjugate intake pls visit:
https://brainly.com/question/32676227
#SPJ11
best answer gets marked as brainliest! list all of the properties of stainless steel spoon
Answers
Answer:
- Corrosion resistant
- High tensile strength (the maximum stress that a material can withstand while being stretched or pulled before breaking)
- Temperature resistant
- Easy formability and fabrication
- Low maintenance (long lasting)
- Attractive appearance
- Environmentally friendly (recyclable)
Answer:
See below
Explanation:
I suppose you could include these properties:
Specific heat = .5 J/g-C
Density =~ 7500 kg/m^3
melting point approx 1500 ° C
among others .....
What trend in electronegativity do you see as you go across a period/row on the table? What causes this trend?
Answers
Answer: It decreases as it goes down and increases as it goes to the right.
Explanation: This is because as it goes down, the elements are becoming bigger which makes the distance from the proton and electron to become further, causing the attraction to weaken. While as it goes to the right across a period, it increases because the number of protons increase and we know that if there are more protons, the pull of the electrons from the nucleus becomes stronger.
What is true of gas?
Answers
Answer:
Gas is a state of matter that has no fixed shape and no fixed volume. Gases have lower density than other states of matter, such as solids and liquids. There is a great deal of empty space between particles, which have a lot of kinetic energy. ... The particles exert more force on the interior volume of the container
Explanation:
Answer:
Gas is a state of matter that has no fixed shape and no fixed volume. Gases have lower density than other states of matter, such as solids and liquids. There is a great deal of empty space between particles, which have a lot of kinetic energy. ... The particles exert more force on the interior volume of the container.
Explanation:
Give the long configuration for Uranium _
WRITE ANSWER WITH THE FOLLOWING FORMAT
1s2 2s2.........etc
Give the long configuration for Uranium
WRITE ANSWER WITH THE FOLLOWING FORMAT
1s2 2s2.........etc
Answers
The electron configuration for Uranium (U) is based upon the placement of uranium in the fourth column of the actinide series or f block. Therefore the electron configuration for uranium must end as 5f4,
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4e14 5d10 6p6 7s2 5f4
This notation can be written in core notation or noble gas notation by replacing the : 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4e14 5d10 6p6 with the noble gas [Rn].
[Rn] 7s2 5f4
I hope this was helpful.
Match the pictures -
element
mixture
compound
?



Answers
Answer:
Explanation:
1. It is an element since it has only one type of atom
2. It is a compound because 2 types of atoms are chemically bound
3. It is a mixture because it has 2 types of atoms which aren't bound
Answer:
First picture: Element
Second picture: Compound
Third picture: Mixture
Explanation:
First picture:
It represents an element as all the atoms are the same.
Second picture:
The other type of atoms are shown as chemically bonded to the green atoms — in the diagram the green atoms and pink atoms are touching.
Third picture:
It is representing a mixture as the other atoms are not chemically bonded together with the green atoms — the green and orange atoms are seperate and are just mixed
Consider the chemical equation. 2h2 o2 right arrow. 2h2o what is the percent yield of h2o if 87.0 g of h2o is produced by combining 95.0 g of o2 and 11.0 g of h2? use percent yield equals startfraction actual yield over theoretical yield endfraction times 100.. 56.5% 59.0% 88.5% 99.7%
Answers
The percent yield of H₂O, if 87.0 g of H₂O is produced by combining 95.0 g of O₂ and 11.0 g of H₂ is 87.87%.
How do we calculate mass from moles?Mass of any substance will be calculated by using their moles as:
n = W/M, where
W = given or required mass
M = molar mass
Moles of 95g of Oxygen (O₂) = 95g / 32g/mol = 2.96 moles
Moles of 11g of hydrogen (H₂) = 11g / 2g/mol = 5.5 moles
Given chemical reaction is:
2H₂ + O₂ → 2H₂O
From the stoichiometry of the reaction, it is clear that:
1 moles of O₂ = reacts with 2 moles of H₂
2.96 moles of O₂ = reacts with 2×2.96=5.92 moles of H₂
Here hydrogen is the limiting reagent as it has lower moles and formation of water depends on this only.
2 moles of H₂ = produces 2 moles of water
5.5 moles of H₂ = produces 5.5 moles of water
Mass of 5.5 moles of water will be calculated as:
W = (5.5mol)(18g/mol) = 99g
Given theoretical yield of water = 87g
% yield of water will be calculated as:
% yield = (87 / 99)×100 = 87.87%
Hence required value is 87.87%.
To know more about % yield, visit the below link:
https://brainly.com/question/25996347
Answer:
D
Explanation:
Which characteristics of elements are most important to determine the type of compounds they will form? Justify your answer.
Answers
Answer:
The number of valence electrons
It determines the elements reactivity and how it will react with another element to form compounds or molecule.
A basic amino acid has an R group that contains
A) a methyl group
B) a thiol group
C) an amine group
d) a carboxyl group
Answers
A basic amino acid has an R group that contains ( D) a carboxyl group.
What is acid?Acid is a substance that has a pH level of lower than 7.0 and is capable of corroding or dissolving other substances. It is usually found in aqueous solutions and is a highly reactive substance. Examples of acid include sulfuric acid, hydrochloric acid, nitric acid and acetic acid. These are used in a variety of industries such as food production, industrial cleaning and chemical engineering. Acid is also used in the laboratory for titrations, pH testing and other experiments. Acids can be dangerous if mishandled and can cause skin, eye and respiratory irritation and even chemical burns.
To learn more about acid
https://brainly.com/question/26855500
#SPJ1
Which atom is a different element than the others
Answers
An element is the simplest form of a substance. Generally, it cannot be simplified or broken down further into smaller particles. An atom is part of an element.
The number of protons in an atom determines what element you have. For instance hydrogen has one proton, carbon has six.