Answers
Answer:
15
Explanation:
Magnesium Acetate Mg(C2H3O2)2
Number of atoms:
Carbon = 4
Hydrogen = 6
Magnesium = 1
Oxygen = 4
Total = 15
Related Questions
PLEASE HELP...
Balance this nuclear reaction by supplying the missing nucleus. Replace each question mark with an appropriate integer or symbol.
Cf98249 + ? ⟶Db105260+410n
Answers
The balanced form of the nuclear equation is as follows; 249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n.
What is a nuclear equation?A nuclear equation is process such as the fission of an atomic nucleus, or the fusion of one or more atomic nuclei and/or subatomic particles in which the number of protons and/or neutrons in a nucleus changes.
According to this question, Californium element is a reactant to produce dubnium and a neutron as products.
However, the law of conservation of mass must be fulfilled by ensuring the mass and atomic numbers of elements in reactant and product side are the same.
249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n
Learn more about nuclear equation at: https://brainly.com/question/13315150
#SPJ1
For electron as a particle, Energy, E=
Answers
For an electron as a particle, E = \(1/2mv^2\)
Electron as a particleFor an electron as a particle, the energy E can be described using the equation:
E = \(1/2mv^2\)
where
This equation represents the kinetic energy of the electron, which is the E is the energy
m is the mass of the electronv is the velocity of the electron.energy associated with its motion.
This equation assumes classical mechanics and does not take into account relativistic effects that become significant at high speeds close to the speed of light.
More on electrons can be found here: https://brainly.com/question/12001116
#SPJ1
A student planned to make copper sulfate crystals from excess copper oxide and dilute sulfuric acid.
The equation for the reaction is:
CuO(s) + H,SO (aq) -, CuSO (aq) + H20(1)
This is the method used.
1. Add 25 cm° of dilute sulfuric acid to a conical flask.
2. Gently warm the dilute sulfuric acid.
3. Add excess copper oxide to the dilute sulfuric acid.
4. Stir the mixture.
5. Heat to evaporate all the water from the mixture.
Suggest two improvements to the method
Explain why each improvement is needed.
A student plans a method to prepare pure crystals of copper sulfate.
The student's method is:
1. Add one spatula of calcium carbonate to dilute hydrochloric acid in a beaker.
2. When the fizzing stops, heat the solution with a Bunsen burner until all the liquid is gone.
The method contains several errors and does not produce copper sulfate crystals.
Explain the improvements the student should make to the method so that pure crystals of copper sulfate are produced.

Answers
The student's method for preparing pure crystals of copper sulfate contains errors and does not produce the desired outcome.
Use copper oxide instead of calcium carbonate: The student should add copper oxide (CuO) to the hydrochloric acid instead of calcium carbonate. Copper oxide reacts with hydrochloric acid to form copper chloride, which can then be converted to copper sulfate through a subsequent reaction with sulfuric acid.
Add sulfuric acid to the copper chloride solution: After the copper chloride solution is formed, the student should add sulfuric acid to it. This reaction between copper chloride and sulfuric acid will yield copper sulfate and hydrochloric acid. The student should ensure that the correct stoichiometric ratio is maintained to maximize the yield of copper sulfate crystals.
Crystal formation: The student should allow the solution to cool slowly after the reaction with sulfuric acid. This promotes the formation of larger, well-defined copper sulfate crystals.
Filtration and drying: Once the crystals have formed, the student should filter the solution to separate the solid crystals from the remaining liquid. The filtered crystals should then be thoroughly dried to remove any remaining water, resulting in pure copper sulfate crystals.
By following these improvements, the student can obtain pure crystals of copper sulfate.
For more such questions on copper sulfate visit:
https://brainly.com/question/17439051
#SPJ8
Ways hydrogen can attain stability
Answers
Calculate the minimum volume of oxygen
that is required for the complete combustion
of a mixture of 20cm3 of CO and 25cm3 of
hydrogen.
A. 45 cm3
B. 22.5cm3
C. 20 cm3
D. 10 cm3
Answers
Hope this helps
I need help with this question

Answers
Answer:
I think It's the 2nd "There are two compounds present"
In which orbital below would an electron (on average) be farthest from the nucleus?
a. 1s
b. 2p
c. 4f
d. 3s
e. 3d
Answers
Answer:
4f its the last orbital of the above
According to the Aufbau principle and electronic configuration, orbital which is farthest from the nucleus is 4f.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/13497372
#SPJ2
I need help. Thanks if you helped.


Answers
Answer:
Hi! The answer to the first question is A. Nina hears the sound of her drum. This occurs last because all the other events must occur first in order for Nina to hear the drum. She must hit it, the surface of the drum must vibrate, and the sound waves must travel through the air. Only then will she hear the drum.
The answer to the second question is A. Hertz. The number of vibrations per second, or frequency, is measured in hertz (Hz).
Have a great day! - Mani :)
You titrate 234.0 mL of a 0.444 M sodium hydroxide solution with 100.0 mL of an unknown concentration sulfuric acid solution. What is the molarity of H2SO4? Show work
Answers
The molarity of the sulfuric acid solution used in the titration is 1.038 M.
How to calculate molarity?Molarity is the concentration of a substance in solution, expressed as the number of moles of solute per litre of solution.
Molarity of a solution involved in titration can be calculated using the following expression;
CaVa = CbVb
Where;
Ca = acid concentrationVa = acid volumeCb = base concentrationVb = base volumeAccording to this question, 234.0 mL of a 0.444 M sodium hydroxide solution is titrated with 100.0 mL of an unknown concentration sulfuric acid solution.
100 × Ca = 234 × 0.444
100Ca = 103.87
Ca = 1.038 M.
Learn more about molarity at: https://brainly.com/question/31545539
#SPJ1
4. How many feet are in 3.45 km?
Answers
Answer:
11318 feet and 10.771 inches
Explanation:
there is 3280.84 in one Km
just do 3280.84x3.45
Using the following chemical equation and your vast knowledge of stoichiometry, calculate the exact molarity of the NaOH. Hint—it should be around 0.1 M.KHP(aq) + NaOH(aq) NaKP(aq) + H2O(liquid)a)Convert the grams of KHP into moles of KHP using the formula weight of 204.2 grams per mole.b)Convert moles of KHP into moles of NaOH using the stoichiometry given in the above equation.c)Calculate the molar concentration of the NaOH by dividing the number of moles of NaOH calculated immediately above by the volume of NaOH used (measured using the buret). Be sure to convert the volume from mL to L.d)Average the three values obtained to determine the average molar the concentration of the NaOH. GET INFORMATION FROM THE PICTURE
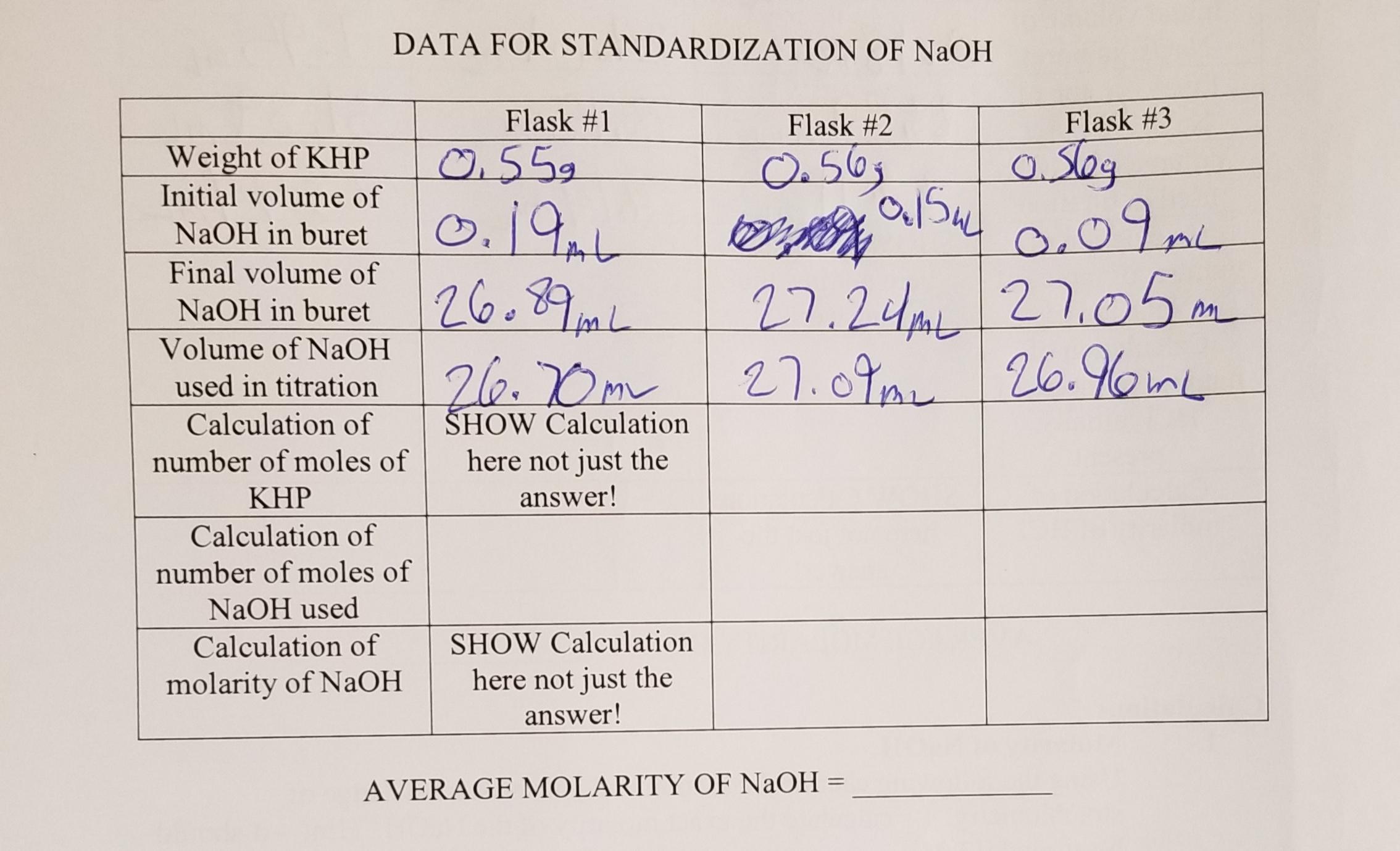
Answers
ANSWER
The molar concentration of NaOH is 0.0705 mol/L
STEP-BY-STEP EXPLANATION:
Given the balanced equation below
\(\text{KHP}_{(aq)}+NaOH_{(aq)}\text{ }\rightarrow NaKP_{(aq)}+H_2O_{(l)}\)According to the balanced equation, 1 mole of KHP gives 1 mole of NaOH
Given parameters
Molar mass of KHP = 204.2 grams/mol
To find the mole of KHP, we will need to find the average grams of KHP used
• For flask 1; 0.55g of KHP was used
,• For flask 2; 0.56g of KHP was used
,• For flask 3; 0.56g of KHP was used
The average mass of KHP used can be calculated below using the average formula
\(\begin{gathered} \text{Average mass = }\frac{mass\text{ 1 + mass 2 + mass 3}}{3} \\ \text{Average mass = }\frac{0.55\text{ + 0.56 + 0.56}}{3} \\ \text{Average mass = }\frac{1.166}{3} \\ \text{Average mass = 0.3886 grams} \end{gathered}\)The average mass of KHP used is 0.3886grams
\(\begin{gathered} \text{Mole = }\frac{\text{ reacting mass}}{\text{molar mass}} \\ \text{reacting mass = 0}.3886\text{ grams} \\ \text{Molar mass = 2}04.2\text{ grams/mol} \\ \text{Mole = }\frac{0.3866\text{ }}{204.2} \\ \text{Mole of KHP = 0.0019 mole} \end{gathered}\)The mole of KHP is 0.0019 mole
PART B
According to the balanced equation, the stoichiometry ratio of KHP to NaOH is 1: 1
Let the mole of NaOH be x
\(\begin{gathered} 1\text{ : 1 = }0.0019\text{ : x} \\ \frac{1}{1}\text{ = }\frac{0.0019}{x} \\ \text{Cross multiply} \\ 1\cdot\text{ x = 1 }\cdot\text{ 0.0019} \\ x\text{ = 0.0019 mole} \end{gathered}\)Hence, the mole of NaOH is 0.0019 mole
PART C
Given the following parameters
0. The volume of NaOH used in flask 1 = 26.70mL
,1. The volume of NaOH used in flask 2= 27.09mL
,2. The volume of NaOH used in flask 3 = 26.96mL
The next step is to convert the mL to L
\(1mL\text{ = 0.001L}\)For flask 1
\(\begin{gathered} 1mL\text{ = 0.001L} \\ \text{Let x be the volume of NaOH in L} \\ \text{ 1mL = 0.001L} \\ 26.70mL\text{ = xL} \\ \text{Cross multiply} \\ xL\cdot\text{ 1ml = 26.70mL }\cdot\text{ 0.001L} \\ x\text{ = }\frac{26.70\cdot\text{ 0.001}}{1} \\ x\text{ = 0.0267L} \end{gathered}\)Using the same conversion process
The volume of NaOH in L in flask 2 = 0.02709L
The volume of NaOH in L in flask 3 = 0.02696L
Hence, the molar concentration of the solution in each flask can be calculated as follows
\(\text{Molar concentration = }\frac{concentration}{\text{Volume}}\)For flask 1
Mole of NaOH = 0.0019 mole
Volume of NaOH = 0.0267L
\(\begin{gathered} \text{Molar concentration = }\frac{0.0019}{0.0267} \\ \text{Molar concentration = }0.0711\text{ mol/L} \end{gathered}\)For flask 2
Mole = 0.0019 mole
Volume = 0.02709L
\(\begin{gathered} \text{Molar concentration = }\frac{Concentration}{\text{volume}} \\ \text{Molar concentration = }\frac{0.0019}{0.02709} \\ \text{Molar concentration = 0.0701 mol/L} \end{gathered}\)For flask 3
Mole = 0.019mole
Volume = 0.02696 L
\(\begin{gathered} \text{Molar concentration = }\frac{concentration}{\text{volume}} \\ \text{Molar concentration = }\frac{0.0019}{0.02696} \\ \text{Molar concentration = 0.0704 mol/L} \end{gathered}\)PART D
Average molar concentration can be found using the below formula
\(\begin{gathered} \text{Average molar concentration = }\frac{0.0711\text{ + 0.0701 + 0.0704}}{3} \\ \text{Average molar concentration =}\frac{0.2116}{3} \\ \text{Average molar concentraion = 0.0705 mol/L} \\ \text{The average molar concentration of NaOH is 0.0705 mol/L} \end{gathered}\)What is the definition of specific heat?
A. The heat needed to raise the temperature of 1 g of a substance
1°C
B. The total amount of energy contained within 1 mole of a
substance
C. The heat required to break the molecular bonds within a
substance
D. The temperature change between the melting and boiling points of
a substance
Answers
Answer:
A. The heat needed to raise the temperature of 1 g of a substance 1°C.
Explanation:
how this has helped you
Explain how balancing
chemical equations with
coefficients conserve the
mass in a reaction.
Answers
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Bohr's Model of the Atom
An element in the alkaline earth metals family has three rings in its Bohr diagram. What is this element?
Answers
Answer:
Magnesium (\({\rm Mg}\).)
Explanation:
In the Bohr Model of atoms, each "ring" denotes a non-empty main energy shell. The Bohr Model for this atom has three rings, meaning that three of the main energy shells of this atom contain electron(s).
Refer to a copy of the modern periodic table. Elements on the modern periodic table are arranged into rows ("periods") by the number of non-empty main energy shells in each atom.
There are three non-empty main energy shells in the atom in this question. Thus, the element of this atom would belong to the third period (third row) of the modern periodic table.
Examples of alkaline-earth metal elements include Beryllium, Magnesium, Calciums, and a few others. These elements are in IUPAC group \(2\) and are found in the second column from the left of a modern periodic table.
There is only one alkaline-earth metal in each period. The question implied that the element here includes three non-empty main energy shells per atom. That element would thus be in the third period. The alkaline-earth metal of the third period is magnesium. Therefore, the element in this question would be magnesium.
The alcoholic, blue solution from Part I of your experiment is commonly used in weather-forecasting devices found in coastal areas of the USA. Based on your observations in the lab explain how this reaction can indicate coming rain
Answers
Answer:
The reaction referred to in this question is likely the reaction between hydrated copper(II) sulfate and anhydrous copper(II) sulfate, where the former is blue and the latter is white.
When the blue solution of hydrated copper(II) sulfate is exposed to moist air, it slowly turns white as water is absorbed, forming anhydrous copper(II) sulfate. This reaction is exothermic, meaning it releases heat, and is reversible. The reverse reaction occurs when anhydrous copper(II) sulfate is exposed to water vapor in the air, forming hydrated copper(II) sulfate and releasing heat.
In coastal areas, the humidity in the air tends to increase before a storm, which can trigger the reverse reaction between anhydrous copper(II) sulfate and water vapor. This releases heat, causing the weather-forecasting device to warm up, indicating that rain may be on the way.
Therefore, the observation of the blue solution turning white in the lab, which indicates the reversible reaction between hydrated copper(II) sulfate and anhydrous copper(II) sulfate, can indirectly indicate the presence of moisture in the air and the possibility of rain, similar to the process in weather-forecasting devices.
What ion has a +3 charge, 28 electrons and an atomic mass of 71?
Answers
The ion with a +3 charge, 28 electrons, and an atomic mass of 71 is the aluminum ion (\(Al^{3+}\)).
Aluminum (Al) typically has an atomic number of 13, which means it has 13 protons and 13 electrons in its neutral state. However, in the given ion, \(Al^{3+}\), the ion has lost three electrons, resulting in a +3 charge. This means that the ion now has 13 protons and only 10 electrons remaining, giving it a net positive charge of +3.
The atomic mass of aluminum is 26.98 atomic mass units (amu). The given ion has an atomic mass of 71 amu, which suggests that the ion has gained additional particles. In this case, the ion has also gained three neutrons, resulting in a higher atomic mass.
The total number of particles (protons, neutrons, and electrons) in the ion can be calculated by adding the number of protons (13) and the number of neutrons (3), which equals 16. Since the ion has a net charge of +3, it only contains 10 electrons.
In summary, the ion with a +3 charge, 28 electrons, and an atomic mass of 71 is the aluminum ion (\(Al^{3+}\)), which has 13 protons, 10 electrons, and 3 neutrons.
for such more questions on electrons
https://brainly.com/question/26084288
#SPJ8
Write the net ionic reaction for the following double replacement reaction:
CuSO4(aq) + Na2S(aq) → CuS(s) + Na2SO4(aq)

Answers
Net ionic equation
Cu²⁺(aq)+S²⁻(aq)⇒CuS(s)
Further explanationDouble-Replacement reactions. Happens if there is an ion exchange between two ion compounds in the reactant to form two new ion compounds in the product
In the ion equation, there is a spectator ion that is the ion which does not react because it is present before and after the reaction
When these ions are removed, the ionic equation is called the net ionic equation
For gases and solids including water (H₂O) can be written as an ionized molecule
Reaction
CuSO₄(aq)+Na₂S(aq)⇒CuS(s)+Na₂SO₄
ionic equation
Cu²⁺(aq)+SO₄²⁻(aq)+2Na⁺(aq)+S²⁻(aq)⇒CuS(s)+2Na⁺(aq+SO₄²⁻(aq)
spectator ions : 2Na⁺ and SO₄²⁻
Net ionic equation
Cu²⁺(aq)+S²⁻(aq)⇒CuS(s)
How does the number of atoms or molecules in a system affect its thermal energy?
A. A system with fewer atoms and molecules has more thermal energy.
B. A system with more atoms and molecules has more thermal energy.
C. The number of atoms or molecules does not affect the thermal energy of a system.
D. Thermal energy increases as the atoms and molecules in a system move more.
Answers
Answer:
D. Thermal energy increases as the atoms and molecules in a system move more.
Explanation:
Thermal energy is a form of kinetic energy possessed by molecules of a system. The measure of this kinetic energy in an atom is called heat.
The average kinetic energy of a system is the temperature.
According to the kinetic theory, the more the particles move, the more their thermal energy. Thermal energy is often predicated on the velocity of the particles of the medium.A reaction between
a
base and an ammonium salt
Answers
Answer:
here's your answer..
Explanation:
mark me brainliest

what is the mass of one sheet of paper in (g) of 500 sheets is 5 pounds? show in conversion factor
Answers
Answer:
1/5
Explanation:
1/5×500
=so guess this is the one
Helium is more reactive than magnesium.
TRUE
FALSE
Answers
Answer: FALSE
Explanation: Magnesium is more reactive.
Hope this helps!
Answer:
False
Explanation:
Magnesium is more reactive.
In the chemical equation CH4 + O2 -> CO2 + 2H2O for every one mole of carbon dioxide you produced, how many moles of water would you have?
Answers
Answer: 2 moles
Explanation:
In the balanced equation, it can be seen that everything reacts in a 1:1 mol ratio except water (H2O) as determined by the coefficient. It is simple stoichiometry, 1 mol CO2 * 2 mol H2O/1 mol CO2 = 2 mol CO2. After doing the math, the CO2 cancels out, leaving how many moles of H2O there are.
The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction.
a. True
b. False
Answers
Answer:
True
Explanation:
In a cell reaction, half cell reaction is oxidation. Oxidation means addition of electrons. This addition of electrons takes place at cathode. Therefore, cathode is site of oxidation. However, the other half cell reaction is reduction. In reduction reaction removal of electrons takes place. Reduction takes place at anode of the cell.
Therefore, the given statement is true.
(07.02 LC)
Which of the following statements is true about the specific heat capacity of a
substance?
It is higher for good conductors.
It is an intensive physical property.
It depends on the melting point of substance.
It depends on the amount of substance.

Answers
Answer:
It is an intensive physical property.
Explanation:
I searched it up
The true statement related to the specific heat capacity of a substance is an intensive physical property.
What is specific heat capacity?The heat capacity should based on the mass of the substance and it have the extensive property.
However, specific heat is the heat capacity per unit mass and it should create the independent of substance amount.
So we can say that specific heat is the intensive property.
Hence the second option is correct.
learn more about heat here; https://brainly.com/question/19514472
Aspirin synthesis involves the addition of an acetyl group to salicylic acid in a condensation reaction with an alcohol. The acetyl group could be added with a carboxylic acid but the preferred procedure is to use the acid anhydride. Why is preferable to use an acid anhydride for ester formation with an alcohol rather than a carboxylic acid
Answers
Answer:
See explanation
Explanation:
The synthesis of asprin involves the;
i) Hydrolysis of Methyl salicylate
ii) Reaction of salicylic acid with acid anhydride
The chemical name of asprin is acetylsalicylic acid.
It is preferable to use an acid anhydride for ester formation with an alcohol rather than a carboxylic acid because we will not produce water which can then cause the hydrolysis of the newly formed ester.
A gold necklace has a mass of 25.6grams and a volume of 1.28cubic centimeters. Calculate its density.
Answers
Answer:25.75
Explanation:
Imagine you are sitting on a surfboard in the ocean, waiting to catch a big wave. When the ocean is still, you are sitting at a point that would be a midpoint (the undisturbed position) of any waves that come your way. You look out to see and see a set of waves coming your way. What you see is actually a pattern of troughs followed be crest of the waves moving forward you and the shore. The distance between the crest is 5 meters. A crest passes you every 20 seconds.
Answer these questions:
A. What is the wavelength?
B. What is the frequency?
C. What is the wave velocity?
Plzzz help with is for a Science assignment so plz put a good answer the person who answers this good gets brainly!
Answers
Answer:
a. What is the wavelength?
The wavelength is 5 meters.
b. What is the frequency?
The frequency is 0.05Hz
c. What is the wave velocity?
The wave velocity is 0.25 meters per second
Explanation:
the wavelength is the distance between two troughs or two crests, which in this case is 5 meters.
the frequency=cycles/time. For this equation the equation would be 1/20, which is 0.05
the wave velocity=wavelength x frequency. Plug in the numbers, 5 x 0.05, and you will get 0.25.
How can a healthcare professional reassure a patient nonverbally?
Answers
Answer:
A.
by explaining the patient’s chart
B.
by nodding at what the patient says
C.
by multitasking while speaking with the patient
D.
by writing down instructions for the patient to follow
Are the options.
Explanation:
what the answer to this

Answers
Compared to the freezing point and boiling point of water at 1 atmosphere, a solution of salt and water at 1 atmosphere has a option b. lower freezing point and a higher boiling point.
When a solute, such as salt, is added to water, it affects the properties of the solution, including its freezing and boiling points. Adding salt to water lowers the freezing point and raises the boiling point compared to pure water at the same pressure.
Lower Freezing Point: The presence of salt disrupts the formation of ice crystals, making it more difficult for the water molecules to arrange into a solid structure. This results in a lower freezing point for the saltwater solution compared to pure water.
Higher Boiling Point: The dissolved salt particles increase the boiling point of the solution. It requires more energy to break the intermolecular forces between the water and salt molecules, resulting in a higher boiling point for the saltwater solution compared to pure water.
Therefore, option B is correct: a solution of salt and water at 1 atmosphere has a lower freezing point and a higher boiling point compared to pure water.
Know more about boiling point here:
https://brainly.com/question/30282615
#SPJ8
The correct answer is :
Compared to the freezing point and boiling point of water at 1 atmosphere, a solution of salt and water at 1 atmosphere has a
A. lower freezing point and a lower boiling point.
B. lower freezing point and a higher boiling point.
C. higher freezing point and a lower boiling point.
D. higher freezing point and a higher boiling point.