how is chromatography demonstrated by using filter paper
Answers
Answer:
When a colored chemical sample is placed on a filter paper, the colors separate from the sample by placing one end of the paper in a solvent.
Related Questions
what did you observe while benzoic acid-salt mixture is melting?
Answers
what did you observe while benzoic acid is melting?
I observed while benzoic acid is melting it bubbling
PLEASE ANSWER QUICK 20 PTS AND ILL MARK YOU BRAINLIEST
What is Sodium (Na) level of reactivity?
Low Reactivity
Medium Reactivity
High Reactivity
Not Reactive- Stable
Answers
What are some chemical changes in hydrogen?
Answers
Hydrogen changes from a gas to a liquid at a temperature of -252.77°C (-422.99°F) and from a liquid to a solid at a temperature of -259.2°C (-434.6°F). It is slightly soluble in water, alcohol, and a few other common liquids.
The table below lists the properties of a metallic element. Shiny, Silver colored, Forms +1 and +2 ions, compound with sulfur is bright red. Where on the periodic table would this element most likely be found?
Group 1
Group 2
Group 12
Group 13

Answers
Answer:
The correct option is;
Group 12
Explanation:
A metallic element that is shiny and silver colored that can exist in the +1 and +2 ionic states and which forms a bright red compound with sulfur is mercury, Hg
The compound formed between mercury and silver is one of the earliest synthetic compound also known as vermilion and cinnabar. The bright red pigment of the HgS is used widely and is one of the most favorite pigment found in medieval European outstanding works of arts and in Chinese decorated wares made of lacquer as well as in mesoAmerica.
Answer:
12
Explanation:
20 POINTS!!
What is the enthalpy of combustion when 1 mol C6H6(g) completely reacts with oxygen?
2C6H6(g) + 15O2(g) ? 12CO2(g) + 6H2O(g)
Options:
A. - 6339 kJ/mol
B. - 3169 kJ/mol
C. 1268 kJ/mol
D. 6339 KJ/mol

Answers
Answer:
-3169
Explanation:
trust the process
-3169 kJ/mol is the enthalpy of combustion when 1 mol \(C_6H_6(g)\) completely reacts with oxygen.
Explanation:
Given:
The reaction of combustion of \(C_6H_6(g)\) along with enthalpies of formation of the compounds.
To find:
The enthalpy of combustion when 1 mol \(C_6H_6(g)\) completely reacts with oxygen.
Solution:
\(2C_6H_6(g) + 15O_2(g) \rightarrow 12CO_2(g) + 6H_2O(g)\)
Enthalpy of the reaction:
\(\Delta H^o_{rxn}=\sum [\Delta H^o_{f,products}]-\sum [\Delta H^o_{f,reactants}]\\=[12mol\times \Delta H^o_{f.CO_2(g)}+6mol\times \Delta H^o_{f,H_2O(g)}]-[2 mol\times \Delta H^o_{f.C_6H_6(g)}+15\times \Delta H^o_{f,O_2(g)}]\\=[12mol\times (-393.50 kJ/mol)+6mol\times (-241.82 kJ/mol)]-[2mol\times 82.90kJ/mol+15mol\times 0 kJ/mol]\\=-6338.72 kJ\)
\(2C_6H_6(g) + 15O_2(g) \rightarrow 12CO_2(g) + 6H_2O(g).\Delta H^o_{rxn}=-6338.72 kJ\)
When 1 mole of \(C_6H_6(g)\) reacts with oxygen gas:
\(=\frac{\Delta H^o_{rxn}}{\text{2 mol of } C_6H_6}\\=\frac{-6338.72 kJ}{2 mol}\\=-3169.36 kJ/mol\approx -3169 kJ/mol\)
-3169 kJ/mol is the enthalpy of combustion when 1 mol \(C_6H_6(g)\) completely reacts with oxygen.
Learn more about enthalpy of combustion here:
brainly.com/question/10583725?referrer=searchResults
brainly.com/question/4075722?referrer=searchResults
A fall outbreak of influenza caused many absences at work and school. Health department
officials sent out warnings to use precautions when coughing and to wash hands
thoroughly. Which members of the population are a primary concern?
Answers
Influenza viruses spread mainly through droplets of respiratory secretions in the air, typically generated by coughing and sneezing.
What is Influenza viruses?A contagious illness brought on by influenza viruses, influenza is also referred to as "the flu." Flu-like symptoms, which can range in severity from moderate to severe, frequently include fever, runny nose, sore throat, muscle discomfort, headache, coughing, and exhaustion. After being exposed to the virus, these symptoms usually appear one to four days later and remain for two to eight days.
Especially with children, diarrhea and vomiting can happen. Pneumonia, which can be brought on by the influenza virus or a subsequent bacterial infection, may develop from the viral-related illness. Other side effects of infection include meningitis, encephalitis, acute respiratory distress syndrome, and deterioration of pre-existing conditions like asthma and cardiovascular disease.
To learn more about Influenza viruses from the given link:
https://brainly.com/question/11736176
#SPJ4
a parent nucleus 82x227 decays 6 particles and 5 electrons (e-). what then is the z value for the daughter nucleus y?
Answers
There can be emissions of radiations like gamma radiation. There can be emission of particles too like alpha particle. Therefore, the z value for the daughter nucleus y is 14.5.
What is nuclear decay?Nuclear decay is process in which the radioactive element releases particles or radiations. Alpha particles is ⁴₂He. Alpha particle is nothing but helium particle.
₈₂²²⁷X\(\rightarrow\) 6 Y + 5 ⁰₋₁e
total atomic number on left side= total atomic number on right side
82= 6(atomic number of Y)+5(-1)
82=6(atomic number of Y)-5
87=6(atomic number of Y)
atomic number of Y= 14.5
Therefore, the z value for the daughter nucleus y is 14.5.
To know more about nuclear decay, here:
https://brainly.com/question/21114779
#SPJ1
Choose the correct statements when comparing 1 mole of carbon
monoxide and one mole of helium gas.
a: they weigh the same
b: they have the same amount of particles
c: they occupy the same volume
Answers
Answer: they have the same amount of particles
Explanation:
This statement is true by Avogadro's Law, which states that in a mole of any substance, there are \(6.022 \times 10^{23}\) particles.
if two group in the game are exirting equal ad opposite force the rope,will the rope move?
Answers
Answer:
No.
Explanation:
If the forces are acting on opposite direction, they will cancel out each other. As a result, the object will not move. This type of force is called balanced force. In this type of force the net force acting on an object is equal to 0. Hence, if two groups in the game are exerting equal and opposite force, the rope will not move.
Which group (family) of non-metals does not form ions?
Answers
Answer:
Noble gasses
Explanation:
nobled gasses
all elements want to loose or gain electrons to be like the noble gasses in bonding but the noble gasses do not bond.
►
17.2H2 + O2 – 2H20
How many moles of oxygen are needed if 8 moles H2 are used?
Answers
If the reaction consumes methane gas ( CH4 ) at a rate of 2.07 M/s, what is the rate of formation of H2
Answers
The reaction consumes methane gas (CH₄ ) at a rate of 2.07 M/s, so the rate of formation of H₂.
The chemical equation for the given reaction is:
CH₄ + 2O₂ → CO₂ + 2H₂O
Here, methane gas (CH₄) is reacting with oxygen (O₂ to form carbon dioxide (CO₂) and water (H₂O). The reaction shows that 1 molecule of CH₄ reacts with 2 molecules of O2 to form 2 molecules of H₂O and 1 molecule of CO₂.
Let's calculate the rate of formation of H₂ using the rate of consumption of CH4 given in the question.Rate of consumption of CH4 = 2.07 M/s
According to the balanced chemical equation, 2 moles of H₂O are formed by the reaction of 1 mole of CH4.
So, 4.14 moles of H₂O are formed when 2.07 moles of CH4 is consumed by the reaction.
Hence, the rate of formation of H₂ = rate of formation of H₂O = (4.14/2) M/s = 2.07 M/s
Answer: 2.07 M/s
Learn more about reaction at https://brainly.com/question/25769000
#SPJ11
How does cold water affect the speed of dissolving?
Answers
Answer:
Anything that can be done to increase the frequency of those collisions and/or to give those collisions more energy will increase the rate of dissolving.
Explanation:
depended on the temperature
So they are asking how human activities in nature will affect the availability in synthetic materials

Answers
Dickey created the first artificial materials with memory for a template in 1949 using a silica gel matrix.
What are synthetic material?By using colors as templates and acid precipitating aqueous sodium silicate solution, imprinted silica materials were created (e.g., methyl orange).
The study of imprinted silicates and metal oxide sol-gel continued in the years that followed, and simple amorphous silicates could be imprinted for various colors, N-heterocycle aromatics, proteins, and for resolution of enantiomers.
The gel structure and surface chemistry of these materials, both of which are controlled by a wide range of variables, including catalysts, pH, solvents, age, and the composition of precursors, determine their recognition properties.
Therefore, Dickey created the first artificial materials with memory for a template in 1949 using a silica gel matrix.
To learn more about Synthetic materials, refer to the link:
https://brainly.com/question/24357817
#SPJ1
A2B8 + C2 - B2C + AC2
Answers
Answer:
You need to put more info! If you are trying to find the GCF its 1
how many mlliliters ofa 12.0 m aqueous hno3 solution should you use to prepare 850.0 ml of a 0.250 m hno3 solution
Answers
The amount in milliliters of a 12.0 M aqueous HNO₃ solution you should use to prepare 850.0 ml of a 0.250 M HNO₃ solution is approximately 17.7 mL.
To prepare 850.0 mL of a 0.250 M HNO₃ solution using a 12.0 M aqueous HNO₃ solution, you'll need to use the dilution formula:
M1V1 = M2V2
where M1 is the initial concentration (12.0 M), V1 is the volume of the initial solution needed, M2 is the final concentration (0.250 M), and V2 is the final volume (850.0 mL).
Rearranging the formula to find V1:
V1 = (M2V2) / M1
V1 = (0.250 M × 850.0 mL) / 12.0 M
V1 ≈ 17.7 mL
So, you should use approximately 17.7 mL of the 12.0 M aqueous HNO₃ solution to prepare 850.0 mL of a 0.250 M HNO₃ solution.
Learn more about dilution here: https://brainly.com/question/27097060
#SPJ11
Which of the following is one part of a chemical formula for a molecule?
A) A number that shows the total number of chemical bonds
B) Numbers that show how many atoms of each element are in the molecule
C) A number showing the atomic masses of each element
D) A Lewis dot diagram for the molecule
Answers
24 ml of benzaldehyde treated with 1 g kcn to yield 25 g of benzoin, which was the limiting reagent?
Answers
Benzaldehyde was the limiting reagent in the reaction between benzaldehyde and KCN, resulting in the formation of benzoin.
In order to determine the limiting reagent, we compare the amount of each reactant used to the amount of product obtained. In this case, we are given that 24 mL of benzaldehyde and 1 g of KCN were used in the reaction, and the resulting yield of benzoin was 25 g.
To determine the limiting reagent, we need to calculate the amount of benzoin that would be formed if each reactant were completely consumed. Since the molar mass of benzaldehyde is approximately 106 g/mol and the molar mass of KCN is approximately 65 g/mol, we can calculate the theoretical yield of benzoin for each reactant.
For benzaldehyde:
24 mL of benzaldehyde is equivalent to approximately 24 g (assuming the density of benzaldehyde is close to 1 g/mL).
The theoretical yield of benzoin from 24 g of benzaldehyde would be (25 g/mol) x (24 g) / (106 g/mol) = 5.66 g.
For KCN:
The theoretical yield of benzoin from 1 g of KCN would be (25 g/mol) x (1 g) / (65 g/mol) = 0.385 g.
Comparing the theoretical yields, we can see that the amount of benzoin obtained (25 g) is closer to the theoretical yield calculated from benzaldehyde (5.66 g) than from KCN (0.385 g). Therefore, benzaldehyde is the limiting reagent in this reaction.
Learn more about benzaldehyde here
https://brainly.com/question/14724597
#SPJ11
This process of heat transfer by conduction would NOT work __________
A) in space.
B) in a solid.
C) under water.
D) in the atmosphere.
Science
Answers
Hope dat helped
Answer:
B and C are the correct answers.
when filling a burette for a titration, adjust the burette so that
Answers
Answer:
When filling a burette for a titrant, adjust the burette so that the opening is near or below the eye leve preferably over the sink. Then, use a funnel to add the titrant into the burette. The titrant should be filled almost to the zero mark.
Determine the density of honey. *
Mass = 162 g
Volume = =107-mL
CE
Clover
HONEY
Honey
US Orade
NET WTORIOL
Answers
Answer:
The answer is
1.51 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 162 g
volume = 107 mL
The density is
\(density = \frac{162}{107} \\ = 1.514018691...\)
We have the final answer as
1.51 g/mLHope this helps you
(HELP!) This is a conversion problem (listed in the photo below) that needs work shown. I figured out the correct overall answer, but I'm pretty sure I used calculations that you are not supposed to use when solving it professionally.
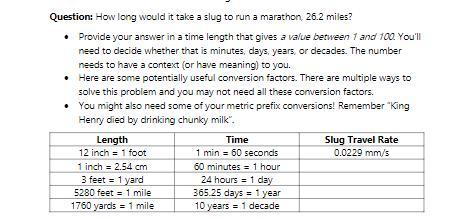
Answers
The using conversion factor the time it takes the slug to run a marathon of 26.2 miles is 148 days
What is conversion factor?Conversion factor is a ratio or factor usaed to convert from one physical units to the other.
How long will it take a slug to run a marathon 26.2 miles?The time it takes the slug to run the marathon is t = d/v where
d = distance and v = speed of slugGiven that
d = 26.2 miles and v = 0.0229 mm/s,Using conversion factor from the table, we convert the distance to mm.
So, d = 26.2 miles
= 26.2 mile × 5280 ft/1 mile × 1 in/12 ft × 2.54 cm/1 in
= 351373.44/12 cm
= 29281.12 cm
= 29281.12 cm × 10 mm/1cm
= 292811.2 mm
So, the time t = d/v
= 292811.2 mm/0.0229 mm/s
= 12786515.2838 s
We now use conversion factor from the table to convert this time to days
t = 12786515.2838 s × 1 min/60 s × 1 h/60 min × 1 day/24 hours = 12786515.2838 s × 1 day/86400 s
= 147.99 days
≅ 148 days
So, using conversion factor the time it takes the slug to run a marathon of 26.2 miles is 148 days
Learn more about conversion factor here:
https://brainly.com/question/24545553
#SPJ1
Conduct research to examine the following factors regarding the storage of nuclear waste.
the costs, risks, and benefits to building a nuclear waste storage facility beneath Yucca Mountain
the costs, risks, and benefits to building a nuclear waste storage facility somewhere else
the costs, risks, and benefits of not building a nuclear waste storage facility at all
Based on the data you have compiled, propose an appropriate solution to this problem. Use your data to support your position on the issue.
Answers
In order to reduce the risk of radiation exposure to individuals and environmental contamination, radioactive wastes are kept. The wastes' radioactivity decreases over time.
What are the biggest problems with keeping radioactive waste in storage for a long time?Large steel and concrete barrels that contain the garbage are typically properly sealed, although accidents and leaks can still happen. Cancerous growths can result from the severe negative impacts of nuclear waste on life.
How is radioactive waste stored?Currently, dry casks are used to store all of the nuclear waste that a power plant produces over the course of its lifetime. Since 1987, Yucca Mountain in Nevada has been intended as a permanent disposal location for spent nuclear material.
To know more about radioactive wastes visit :-
https://brainly.com/question/9816140
#SPJ1
Many of the lunar craters are due to volcanic eruptions true or false
Answers
Answer:
false
Explanation:
Craters on the Moon are caused by asteroids and meteorites colliding with the lunar surface. The Moon's surface is covered with thousands of craters. ... It also has very little geologic activity (like volcanoes) or weathering (from wind or rain) so craters remain intact from billions of years.
Which of these is an accurate description of convection

Answers
Answer:
The movement of materials based on differences in temperature and density, I believe
Explanation:
Please help fast! 20 points.

Answers
When we bring a magnet near the doorbell when it is not connected to the battery, we feel a pull, or an attractive force.
For this the hypothesis can be:
Hypothesis: If there is no permanent magnet in the doorbell, just metal like iron, then when we bring a paper clip to the doorbell, we will observe an attractive force between the paper clip and the doorbell due to the interaction between the magnet and the iron in the doorbell.
Hypothesis: If there is a permanent magnet in the doorbell, then when we bring a paper clip to the doorbell, we will observe a stronger attractive force between the paper clip and the doorbell due to the interaction between the magnet and the metal components (such as iron) in the doorbell.
Thus, these can be the Hypothesis for the given scenario.
For more details regarding Hypothesis, visit:
https://brainly.com/question/29576929
#SPJ1
Explain why nervous signals are described as an "electrochemical process." What is electrical and what is
chemical? Make sure to use these terms (axon, synapse, neurotransmitter, action potential, Na/k pump, dendrite, axon
terminals).
Answers
Answer:
The nervous system operates using an electrochemical process. An electrical charge moves through the neuron itself, and chemicals are used to transmit information between neurons
Answer: The nervous system operates using an electrochemical process. An electrical charge moves through the neuron itself, and chemicals are used to transmit information between neurons
Explanation: i just took the test
Which rule or principle does this violate?
A. Aufbau Principle
B. Pauli Exclusion Principle
C. Hunds rule

Answers
Answer:
Hunds rule is violated here.
Hunds rule : pairing of electrons takes place only if all the orbitals are filled with single electrons each.
How many protons, neutrons, and electrons are present in an atom of zinc, Zn, with a mass
number of 65?
Answers
Group number : 12
Period number : 4
Block : d block
Element : Transition elements.
Part 2:
Protons in an atom of Zn: 30
Part 3:
Electrons in a Zn atom: 30
Part 4 :
Neutron in an atom of Zn: 35
The pH of the ocean is around 8.1, is the ocean considered a
buffer? Why or Why not?
Answers
Yes, the sea is considered a buffer.
A buffer is a solution that resists pH changes when acids or bases are added. The buffering capacity of the ocean allows it to maintain a relatively stable pH even when acids and bases are added.
The ocean's buffering capacity is primarily due to the presence of dissolved compounds such as bicarbonate (HCO3-) and carbonate (CO32-). These compounds act as both weak acids and bases, accepting and releasing hydrogen ions (H+) to maintain pH balance. When carbon dioxide (CO2) in the atmosphere dissolves in seawater, carbonic acid (H2CO3) is produced and decomposed into bicarbonate ions and hydrogen ions.
This transformation helps prevent a rapid drop in pH as excess hydrogen ions combine with carbonate ions to form bicarbonate ions, which can reduce overall acidity.
When alkali such as hydroxide ions (OH-) is added to the ocean, excess hydroxide ions combine with hydrogen ions to form water molecules, reducing alkalinity.
The presence of these dissolved compounds and their interconversion reactions stabilize the pH of the ocean, making it less susceptible to rapid changes in acidity or alkalinity. This buffering capacity is essential for the survival and maintenance of marine life, as many organisms are sensitive to changes in pH.
To know more about PH refer to this link
https://brainly.com/question/12609985