how does one know that khso5, the active reagent in oxone, is a strong oxidant?
Answers
The reactivity of oxone in the oxidation of organic molecules depends on the reaction conditions as well as the chemical structure of the organic molecule being oxidized. However, it is known that KHSO5 is a powerful oxidant with several mechanisms of oxidation.
The oxone (KHSO5) is a triple salt, consisting of 2 equivalents of potassium hydrogen sulfate and 1 equivalent of potassium sulfate. Oxone is an oxidant that is very strong and effective in the oxidation of a variety of organic molecules. The main reason why oxone is a strong oxidant is that it has a unique mechanism of oxidation.In the case of KHSO5, the sulfur(VI) species is capable of undergoing a series of electron transfers that generate a number of highly reactive oxygen species.
For example, the sulfur(VI) species can undergo a reaction with water to form the highly reactive peroxymono sulfate anion (HSO5-). The peroxymono sulfate anion is highly reactive due to the presence of a labile oxygen-oxygen bond, which is susceptible to nucleophilic attack by a variety of organic molecules .In addition to peroxymono sulfate, the sulfur(VI) species can undergo a reaction with hydrogen peroxide to form the peroxodisulfate dianion (S2O82-).
To know more about oxidized visit:
https://brainly.com/question/13182308
#SPJ11
Related Questions
Nitric oxide, NO, is made from the oxidation of NH3, and the reaction is represented by the equation:4NH3 + 5O2 → 4NO + 6H2OWhat mass of O2 would be required to react completely with 7.42 g of NH3?
Answers
• Molar mass NH3 ,= Molmass of N + ( 3xmol.mass of H ) = ,17 g/mol
,• Molar mass of O2, = 2 x Mol.mass of O = 2*16 = ,32 g/mol
,• Mass of NH3 = 7.42 g
The balanced equation of the chemical reaction:
\(4NH_3+5O_2\Rightarrow4NO+6H_2O\)According to stoichemistry ,
• 4 moles of NH3 reacts with 5 moles of O2
,• 4x17 g of NH3 reacts with 5x32 g of O2
• then , 7.42 g NH3 reacts with x mass of O2
Therefore mass of Oxygen will be :\(\begin{gathered} MassO_2\text{ = }\frac{7.42\cdot\text{ 5 }\cdot32}{4\cdot17} \\ \text{ = }17.45\text{ g } \end{gathered}\)This means that mass of Oxygen required = 17.45 gexplain what a convection current is?
who ever sees this I hope you have a wonderful day!
Answers
Answer:
Convection currents are flowing fluid that is moving because there is a temperature or density difference within the material.
Because particles within a solid are fixed in place, convection currents are seen only in gases and liquids. A temperature difference leads to an energy transfer from an area of higher energy to one of lower energy.
Please help me on this

Answers
..
Calculate the amount of energy required to boil 25.00 g of mercury.

Answers
Answer:
C. 7.4 kJ
Explanation:
Let assume that Mercury is at room temperature (25 °C). The energy required to boil the sample of mercury is the sum of sensible and latent heats. Mercury has a fussion and boiling points of -38.83 °C and 356.7 °C, respectively, and a specific heat of \(138\,\frac{J}{kg\cdot ^{\circ}C}\). Then:
\(Q = (25\,g)\cdot \left[\left(0.138\,\frac{J}{g\cdot ^{\circ}C} \right)\cdot (356.7^{\circ}C - 25^{\circ}C) + 296\,\frac{J}{g} \right]\)
\(Q = 8544.365\,J\)
The option that offers the best approximation to the result is C.
162.4 NH3 =____mol NH3
Answers
Answer:
162.4
Explanation:
Because yea thats the answer no need to tell you why ok bye
How many molecules are there if you have 25.6 grams if titanium (IV)
oxide, used to color paint?
Answers
Answer:
itsball wrong
Explanation:
beucaer
Given 5 grams of H2, how many grams of
CH4 are produced?
Answers
Given 5 grams of H₂, 80 grams of CH₄ are produced by From the equation, we can see that for every 2 moles of CH₄, 1 mole of H2 is consumed.
To determine the amount of CH₄ produced from 5 grams of H₂, we need to consider the balanced chemical equation for the reaction between H₂ and CH₄. From the equation stoichiometry, we can calculate the stoichiometric ratio between H₂ and CH₄ and use it to find the mass of CH₄ produced.
The balanced chemical equation for the reaction between H₂ and CH₄ is:
H₂ + 2CH₄ → 4H₂ + C
To find the mass of CH₄ produced, we need to convert the mass of H2 to moles using its molar mass (2 g/mol) and then use the stoichiometric ratio to calculate the moles of CH₄ produced. The molar mass of CH₄ is 16 g/mol.
First, we convert the mass of H₂ to moles:
5 g H₂ * (1 mol H2 / 2 g H₂) = 2.5 mol H2
Since the stoichiometric ratio is 1:2 for H2 to CH₄, we have:
2.5 mol H₂ * (2 mol CH₄ / 1 mol H₂) = 5 mol CH₄
Finally, we convert the moles of CH₄ to grams:
5 mol CH₄ * (16 g CH₄ / 1 mol CH₄) = 80 g CH₄
Therefore, 5 grams of H₂ will produce 80 grams of CH₄.
Learn more about stoichiometry here
https://brainly.com/question/30676346
#SPJ11
NO LINKS PLS HELP
Which weighs more a sealed, half-filled jar of water or that same jar after it is placed in the freezer until the water turns to ice? How do you know the answer without experimenting?
Answers
Answer:
frozen
Explanation:
I would say because when u freeze water it expands and denifys.
A reaction mixture in a 3.620-L flask at a certain temperature initially contains 0.7660g H2 and 96.80g I2 At equilibrium, the flask contains 90.20 g HI
Answers
What is the right answer?

Answers
Answer:
Option C
Explanation:
In Lewis dot structure the valence electrons are represented as dots around the symbol.
For example,
In hydrogen valence electron is only one it can be represented as,
H·
We know that in covalent bonding atoms share their electron to complete the octet. Thus, their Lewis dot structure would be represented as,
H:H
This is the Lewis dot structure of H₂.
In given atom X their are 2 valence electrons (3s²) thus its Lewis dot structure contain two dots around the symbol.
Option C is correct answer because it has only two dots which actually represent valence electrons.
Other options are incorrect because symbol X has more than two electrons/dots.
you are given a colourless solution, it turned blue litmus paper red
Answers
Answer:
The colorless solution is an acid.
If a microwave uses 96kj of energy in 2 minutes, what is the power rating?
Answers
Answer:
2-7
Explanation:
Which of the following is the smaller atom: magnesium or chlorine?
Answers
Answer:
Chlorine atoms are smaller
Explanation:
Magnesium have more electrons than chlorine.
Answer: Chlorine
Explanation: Since chlorine's 17 protons are greater than magnesium's 12 protons, chlorine will have a greater effective nuclear charge to draw chlorine's valence electrons closer to the nucleus and, thus, chlorine is expected to have the smaller atomic radius, while magnesium with the lower effective nuclear charge is expected to have the larger atomic radius.
Silver nitrate also forms a precipitate with nai. What would this precipitate be?.
Answers
When silver nitrate (AgNO₃) reacts with sodium iodide (NaI), a precipitate forms. The precipitate that is produced in this reaction is silver iodide (AgI).
The reaction can be represented by the following balanced chemical equation:
2AgNO₃ + 2NaI → 2AgI + 2NaNO₃
In this equation, two moles of silver nitrate react with two moles of sodium iodide to yield two moles of silver iodide and two moles of sodium nitrate.
Silver iodide is a yellow, crystalline solid that is insoluble in water. The formation of a precipitate occurs because silver iodide has low solubility, meaning it does not readily dissolve in water. Instead, it separates out of the solution as a solid, forming a visible precipitate.
The reaction between silver nitrate and sodium iodide is a double displacement reaction. The silver ions (Ag+) from silver nitrate combine with the iodide ions (I-) from sodium iodide to form solid silver iodide (AgI). The sodium and nitrate ions remain in solution as sodium nitrate (NaNO3).
Precipitation reactions are commonly used in chemical analysis to identify the presence of specific ions in a solution. In this case, the formation of the yellow precipitate of silver iodide confirms the presence of iodide ions in the solution.
To know more about double displacement reaction, refer to the link below:
https://brainly.com/question/13854110#
#SPJ11
the number of_____ determines what kind of element the atom is
Answers
Answer:
protons
Explanation:
The number of protons in the nucleus determines which element an atom is, while the number of electrons surrounding the nucleus determines which kind of reactions the atom will undergo.
Please help
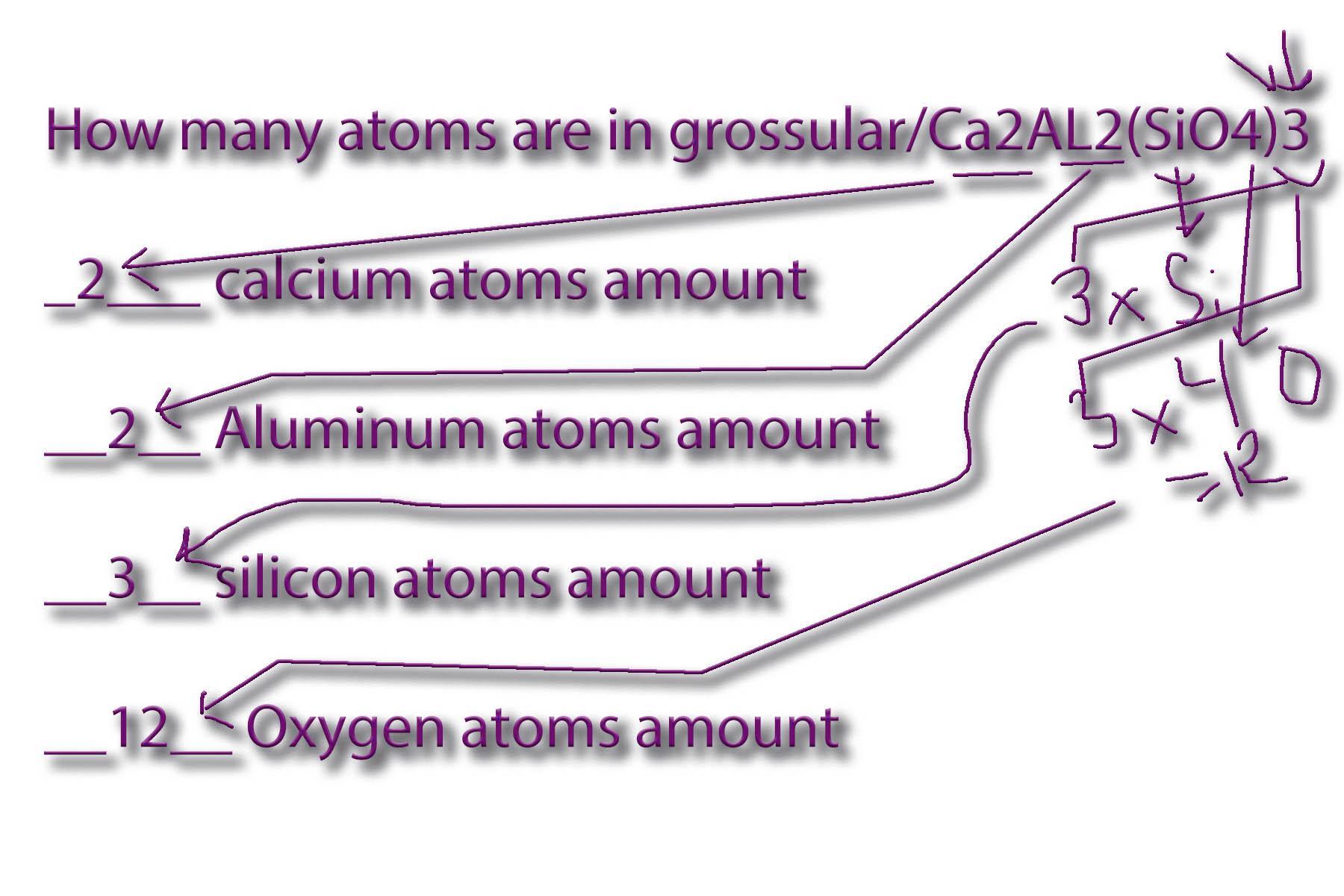
How many atoms are in grossular/Ca2AL2(SiO4)3
____ calcium atoms amount
____ Aluminum atoms amount
____ silicon atoms amount
____ Oxygen atoms amount
Answers
Answer:
See below
Explanation:
How many atoms are in grossular/Ca2AL2(SiO4)3
_2___ calcium atoms amount
__2__ Aluminum atoms amount
__3__ silicon atoms amount
__12__ Oxygen atoms amount
See attached worksheet
A 15.33 g sample of magnesium oxide is found to contain 12.67 g of magnesium. What is the percent (by mass) composition of
the magnesium in the compound?
026 Ma
Answers
Answer:
Explanation:
I can help
Answer:
12.67g of Mg / 15.33g of MgO * 100% = 82.6% of Mg
Explanation:
An atom contains 16 protons, 18 neutrons, and 16 electrons what is its mass number?
Name the element of which this is an isotope.

Answers
16
protons
18
neutrons
16
electrons
Right from the start, you know that you're indeed dealing with a neutral atom, since the number of protons it has in its nucleus is equal to the number of electrons it has surrounding its nucleus.
Now, an atom's atomic number,
Z
, tells you how many protons it has in its nucleus. Nothing more, nothing less.
In your case, you are told that the atom contains
16
protons in its nucleus, which means that
Z
will be equal to
Z
=
16
A quick look in the periodic table will reveal that you're dealing with an atom of sulfur,
S
.
An atom's mass number,
A
, tells you how many protons and neutrons it contains in its nucleus. Since the number of protons is given by
Z
, you can say that
A
=
Z
+
no. of neutrons
In your case, the atom contains
18
neutrons in its nucleus. This means that
A
will be equal to
A
=
16
+
18
=
34
Finally, focus on the atom's electron configuration, which you know that must account for a total of
16
electrons.
S:
1
s
2
2
s
2
2
p
6
3
s
2
3
p
4
Its noble gas shorthand notation, which uses the electron configuration of neon,
Ne
, the noble gas that comes before sulfur in the periodic table, will look like this
S:
[
Ne
]
3
s
2
3
p
4
The body of a victim is discovered in the woods during a week with unusually cold weather. What should a forensic scientist consider when estimating the rate of decomposition of the body tissues of the victim?
The temperature would not need to be considered.
The tissues decompose at a slower rate at lower temperatures.
The tissues decompose at a faster rate at lower temperatures.
The tissues would not decompose at all at a lower temperature.
Answers
Answer:
The tissues decompose at a slower rate at lower temperatures.
Explanation:
This is my best guess but I think answer is tissue decompose slower rate at low temperature. This is why we in medicine we store specimens at very low temperature so we can do research on it at a later time but they eventually go bad. Low temperature slow down enzymes and proteins thus breakdown is slower rate.
if it takes 20.0 ml of 0.050 m h2so4 (aq) to neutralize (reach equivalence point) 8.00 ml of an unknown concentration of lioh(aq), what is the concentration of the lioh? show your work.
Answers
The magnitude of the concentration that LiOH(aq) employs to neutralize the reaction is 0.125 M.
What is the concentration of LiOH(aq)?\(M__{H_{2}SO_4 }\) = M₁ = 0,050 M
\(V__{H_{2}SO_4 }\) = V₁ = 20,0 ml
\(V__{LiOH }\) = V₂ = 8,00 ml
Thus, solving for the final morality is:
M₁V₁ = M₂V₂
M₂ = \(\frac{M_1V_1}{V_{2}}\)
So, plugging in the values we obtain:
M₂ = \(\frac{0,050x20,0}{8,00}\)
M₂ = 1 : 8,00
M₂ = 0,125 M
Then the magnitude of the concentration that LiOH employs to neutralize the reaction is 0.125 M.
Learn more about calculating the concentration of the solution at https://brainly.com/question/19091247
#SPJ4
Explain the trend in boiling points as you move down group v11
Answers
Answer:
Fluorine
Chlorine
Bromine
Iodine
Boiling point increases as you go down the group v11
Explanation:
The elements of Group VII are the halogens consisting of f fluorine (F), chlorine (Cl), bromine (Br), iodine (I). All of which are non metals and exists as diatomic molecules - F2, Cl2, Br2, I2 with intermolecular attractions between the two molecules of each element held by Van der Waals dispersion force.
Moving down the group, the size of the atoms increases in size from Fluorine, F2 and Chlorine, Cl2 which are gases to Bromine , Br2 which exists as a liquid to solid, Iodine, I2. This attributes to the increasing in Strength of the Van der Waals forces as you go down the group. In order to break the vanderwaals forces , More heat energy is required to change thier states leading to the increase in boiling point going down the group.
Fluorine
Chlorine
Bromine
Iodine
Boiling point increases as you go down the group
A nonpolar solvent has the highest solubility with a(n) _____ solute. A. polar B. nonpolar C. ionic D. electrically charged solute.

Answers
Answer:
nonpolar
Explanation:
A nonpolar solvent has the highest solubility with a nonpolar solute.
What does a nonpolar covalent bond show about the electronegativities of its
two atoms?
A. The electronegativity of both of the atoms is zero.
B. The electronegativities of the two atoms are equal.
O c. The difference in electronegativities of the two atoms is very
small.
O D. The difference in electronegativities is greater than 1.7.
Answers
Answer:
B. The electronegativities of the two atoms are equal.
The elements from this section of the periodic table all belong to the same
A. Family
B. Group
C. Period
D. Valence
Plz help!!

Answers
Answer:
C. Period
Explanation:
The elements from this section of the periodic table belongs to the same period.
A period on the periodic table is a horizontal arrangement of elements according to their atomic number.
A group or family is the vertical organization of elements. Valence is the number of outer shell electrons an atom possess.Which statements are true? Check all that apply. Check all that apply. The higher the temperature, the more soluble most ionic solids are in water. As you cool a saturated solution from high temperature to low temperature, solids start to crystallize out of solution if you achieve a supersaturated solution. The higher the temperature, the more soluble most gases are in water. If you raise the temperature of a saturated solution, you can (usually) add more solute and make the solution even more concentrated.
Answers
Answer:
The higher the temperature, the more soluble most ionic solids are in water
As you cool a saturated solution from high temperature to low temperature, solids start to crystallize out of solution if you achieve a supersaturated solution.
If you raise the temperature of a saturated solution, you can (usually) add more solute and make the solution even more concentrated.
Explanation:
For many ionic solids, solubility in water increases with increase in the temperature of the solution.
This implies that increasing the temperature allow more solute to dissolve in the solvent, supersaturation may be achieved by so doing. As the solution is cooled, the solid crystalizes out of solution hence the answers above.
What is the trend in ionization energy as you move across period 2, from li to ne?.
Answers
Ionization energy increases as we move across the period from left to right.
order of ionization energy across period 2
Li < B < Be < C < O < N < F < Ne
What is Ionization energy?
Ionization energy represents the energy required to remove an electron from an isolated gaseous atom (X) in its ground state. It is minimum at the alkali metals and their low ionization enthalpies can be correlated with their high reactivityThe Ionization energy is maximum at the nobel gases since they have closed electron shellsTrends for Ionization energy
There are two trends, the first ionization enthalpy generally increases as we go across a period from left to right and decreases as we go down in a group.Two factors to understand these trends arethe attraction of electrons towards the nucleus and the repulsion of electrons from each other nucleusorder of ionization energy across period 2
Li < B < Be < C < O < N < F < Ne
Be and N are comparitively more stable valence subshell than B and OThe first ionization of Be is greater than that of Boron because Be has a stable complete electronic configuration (1s2 2s2) thus it require more energy to remove the first electron from it, whereas Boron has electronic configuration (1s2 2s2 2p1 ) which need lesser energy than that of Beryllium.Nitrogen has stable electronic configuration of 1s2 2s2 2p3 has half filled p orbital thus it requires more energy to remove an electron from stable valence orbital than oxygen 1s2 2s2 2p4 which need less energyLearn more about Ionization energy at https://brainly.com/question/8980265
#SPJ4
Physics is ______ about
how things move and why things move
Answers
Answer: Physics is about
how things move and why things move
Explanation:
Do you think the offspring of the hydra and the salamander are genetically identical or not genetically identical to the parents? Support your argument with evidence.
Answers
Moreover, sexual reproduction involves combining half of each parent's genetic information to create a unique set of genes in the offspring. Since hydras reproduce through budding (where an individual can produce genetically identical clones), they do not undergo sexual reproduction. On the other hand, salamanders reproduce sexually, so their offspring receive a unique combination of genes from both parents.
Therefore, even if it were possible for a hybridization event between these two organisms to occur (which is highly unlikely due to their biological differences), their offspring would inherit new combinations of genes that are distinct from those found in either parent species; thus making them not genetically identical but rather hybrids with unique genome arrangements reflecting characteristics from both lineages.
anyone know this? I'll brainlist u!

Answers
Answer:
for the second one i'd go with reflection
Explanation:
T or F: sodium chloride as a compound does not truly exist in the ocean.
Answers
The statement is false. Sodium chloride (NaCl) does truly exist in the ocean. In fact, NaCl is the most abundant salt in seawater, making up approximately 85% of all dissolved salts.
The salt in seawater comes from the weathering of rocks on land, which release ions into rivers and ultimately into the ocean. As seawater evaporates, the concentration of NaCl and other salts increases, leading to the formation of salt deposits. Therefore, sodium chloride does truly exist in the ocean.
Sodium chloride (NaCl) is a compound, which means it is a combination of two or more elements. Sodium and chlorine are the two elements that make up sodium chloride. It's also known as table salt or simply salt.
NaCl, or table salt, is present in the oceans, lakes, and other natural bodies of water. It is discovered in huge quantities in seawater, which is roughly 3.5% salt by weight. It's also found in salt deposits underground or in shallow mining operations.
For more question on Sodium chloride click on
https://brainly.com/question/28106660
#SPJ11