How do I write the following :
One atom of Nitrogen
Five atoms of Nitrogen
One molecule of Nitrogen
Five molecules of Nitrogen
One molecule of Sulfur
One atom of Sulfur
Eight molecules of Sulfur
Answers
Answer:
Five molecules of nitrogen and eight molecules of sulfur
Explanation:
Related Questions
Cyclohexane (C6H12) is one of the components of crude oil. Which shows the
balanced combustion reaction for cyclohexane?

Answers
Answer:
D. C6H12+9O2 -> 6CO2+6H2O+heat
Explanation:
got it right on ap ex
The combustion reaction is the reaction between reactants and oxygen. The balanced combustion reaction for cyclohexane is, C₆H₁₂ + 9O₂ → 6CO₂ + 6H₂O + heat. Thus, option D is correct.
What is a combustion reaction?A type of reaction that includes the release of energy and heat when the oxygen reacts chemically with the reactant. The presence of oxygen as one of the reactants is a characteristic property of the combustion reaction.
As a result of the reaction carbon dioxide and water is produced along with heat in the reaction. The balanced combustion reaction of cyclohexane (C₆H₁₂) is given as,
C₆H₁₂ + 9O₂ → 6CO₂ + 6H₂O + heat
Here, the number of carbon is six on both sides, twelve hydrogen on both sides, and eighteen oxygen on the left and right sides of the reaction.
Therefore, option D. C₆H₁₂ + 9O₂ → 6CO₂ + 6H₂O + heat is the balanced combustion reaction.
Learn more about combustion reaction here:
https://brainly.com/question/14335621
#SPJ2
what is the amount of heat required to raise the temperature of 125.0 g of aluminum by 12c? (specific heat of aluminum
Answers
The amount of heat required to raise the temperature of 125.0 g of aluminum by 12°C is 1611 joules.
Temperature = 12°C
Mass = 125.0 g
To estimate the amount of heat required, we need to use the formula:
Q = m * c * ΔT
Q = the amount of heat in joules
m = the mass of the substance in kilograms
c = the specific heat capacity of the substance
ΔT = the change in temperature in degrees
The specific heat capacity of aluminum = 0.897 J/g°C.
Q = 125.0 g * 0.897 J/g°C * 12°C
Q = 1611 J
Therefore, we can conclude that the amount of heat required is 1611 J.
To learn more about the heat required
https://brainly.com/question/3727855
#SPJ4
silver (I) nitrate reacts with nickel (II) chloride to produce silver (I) chloride and nickel (II) nitrate wright the balanced chemical equation for this
Answers
Answer: 2 AgNO3(aq) + NiCl2(aq) ⇒ 2 AgCl(s) + Ni(NO3)2 (aq)
Explanation:
Please help!!!! This is due tomorrow

Answers
Answer:
An ion is a charged atom or molecule. Ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions. Positive ions are called cations and negatively charged ions are called anions.
In your own words, describe what is a scientific theory.
Answers
Answer: Scientific Theory is a is a well-substantiated explanation of something you have repeatedly confirmed through observation and experiment.
answer asap please!!!!!!!!!!!!!!!!!!!!!

Answers
Answer: c.
Explanation: Because it will increase temperature on thermometer y will decease.
How many thumbs would you expect a group of 86 aliens to have? for every 3 aliens there are 8 hands. For every hand there are 2 thumbs
Answers
We would expect a group of 86 aliens to have approximately 458 thumbs. If for every 3 aliens there are 8 hands, then we can find the total number of hands by dividing the number of aliens by 3 and multiplying by 8:
86 aliens / 3 aliens per group * 8 hands per group = 229.33 hands
However, since we are interested in the number of thumbs, we need to multiply the number of hands by 2 (since there are 2 thumbs per hand):
229.33 hands * 2 thumbs per hand = 458.67 thumbs
Since aliens are unlikely to have a fraction of a thumb, we should round down to the nearest whole number.
Therefore, we would expect a group of 86 aliens to have approximately 458 thumbs.
Learn more about thumbs
https://brainly.com/question/15300373
#SPJ4
Question 5 of 10
What is the molecular formula of the compound CH₂ with molar mass = 168
g/mol?
Answers
Answer:
C12H24
Explanation:
The molar mass of the CH2 unit is 14 g/mole. The final molecule has multiples of CH2 so that it's molar mass is 168 g/mole.
(168 g/mole molecule)/(14 g/mole Unit) = 12 Units
12 CH2 units would make the molecualr formula: C12H24
This is more than one structure that has this formula: Cyclododecane and 1-Dodecene.
Is this true Bc I’m not sure
Answers
Answer:
????? questions there no question
Answer:
what
Explanation:
Equal masses of hydrogen, oxygen, and nitrogen gas are all in the same container. Which of the three gases must have the highest partial pressure?.
Answers
The hydrogen gas has the highest partial pressure, which is greater than nitrogen and oxygen gas because hydrogen gas has the smallest molar mass among the three.
The partial pressure of a gas is the pressure that the gas will exert if it alone occupies the same space as the mixture of gases.
Dalton's law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each component gas.
This means that the pressure of each gas in the mixture contributes to the total pressure.
According to Graham's law of effusion and diffusion, the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass.
As a result, since hydrogen gas has the smallest molar mass, it will travel at the highest rate and collide with the container walls more frequently, resulting in a higher partial pressure.
To know more about partial pressure visit;
https://brainly.com/question/13199169
#SPJ11
Are amino acids equivalent to proteins?.
Answers
A protein consists of one or more chains of amino acids (called polypeptides) whose sequence is encoded in a gene. Thus amino acids bind together to form proteins.
All of the body's tissues and organs, including muscle, bone, skin, hair, and every other component or tissue, contain protein. It creates the hemoglobin, which provides oxygen to your blood, and the enzymes that power numerous chemical reactions.
The body uses large, complex molecules called proteins for a number of essential processes. They do the majority of their work in cells.
Amino acids, which are small natural compounds with an alpha (critical) carbon atom coupled to an amino organization, a carboxyl institution, a hydrogen atom, and a variable component known as an aspect chain, are the building blocks of proteins.
To learn more about Amino Acids please click on the given link: https://brainly.com/question/28409615
#SPJ4
Volume of 22mm x 15 mm x 2.0 mm
Answers
The formula for the volume of a rectangular solid is Volume = length * width * height, or V = lwh.
\(volume = 22mm \times 15mm \times 2.0mm \\ = 22mm \times 30mm \\ = 660mm \\ \)Converting to cm = \( \frac{660}{10} = 66 {cm}^{3 } \\ \)
- BRAINLIEST answerer
Identify the calculations possible using only 28.02 g/mol as a conversion factor. Select one or more: Calculate the grams of N2 in 10.58 liters of nitrogen gas Calculate the grams of N2 in 5.03 x 1020 moles of nitrogen molecules Calculate the moles of N2 molecules in 3.94 grams of nitrogen Calculate the moles of N2 molecules in 4.73 liters of nitrogen gas
Answers
Using only 28.02 g/mol as a conversion factor, we can:
Calculate the grams of N₂ in 5.03 × 10²⁰ moles of nitrogen gas.
Calculate the moles of N₂ molecules in 3.94 grams of nitrogen gas.
We want to identify the conversion factors required in a series of calculations:
A conversion factor is an arithmetical multiplier for converting a quantity expressed in one set of units into an equivalent expressed in another.
28.02 g/mol, which is the molar mass of nitrogen, is a conversion factor to convert moles to mass and vice versa.
Calculate the grams of N₂ in 10.58 L of nitrogen gas.
We want to convert 10.58 L (volume) to grams (mass). We need to conversion factors:
22.4 L/mol is the conversion factor to convert volume to moles.
28.02 g/mol is the conversion factor to convert moles to mass.
Calculate the grams of N₂ in 5.03 × 10²⁰ moles of nitrogen gas.
We want to convert 5.03 × 10²⁰ moles (moles) to grams (mass). We can do so by just using 28.02 g/mol as the conversion factor.
Calculate the moles of N₂ molecules in 3.94 grams of nitrogen gas.
We want to convert 3.94 grams (mass) to moles. We can do so by just using 28.02 g/mol as the conversion factor.
Calculate the moles of N₂ molecules in 4.73 L of nitrogen gas.
We want to convert 4.73 L (volume) to moles. The required conversion factor is 22.4 L/mol.
Using only 28.02 g/mol as a conversion factor, we can:
Calculate the grams of N₂ in 5.03 × 10²⁰ moles of nitrogen gas.
Calculate the moles of N₂ molecules in 3.94 grams of nitrogen gas.
Learn more about conversion factor here:
https://brainly.com/question/30166433
#SPJ4
Please help ASAP I’ll mark you as brainlister:(((!!!!!!!!

Answers
Answer:
c. Gas particles don't interact
Explanation:
Ideal gas particles do not interact with each other.
The gas molecules do not interact with each other except for colliding with each other. Gases expand to completely fill a container; they would not if they were attracted to each other. That being said, they obviously still do, as a result of Dalton's Law.
beta decay is nuclear decay in which an electron is emitted from an atom. if the electron is given 0.95 mev of kinetic energy, what is its velocity, as a fraction of the speed of light? you will have to assume the electron is moving relativistically.
Answers
We can use the relativistic energy-momentum relation to solve this problem Therefore, the velocity of the electron is approximately 0.999999954 times the speed of light.
Relativistic effects are observed when the speed of an object approaches the speed of light. At these speeds, the object's kinetic energy and momentum increase significantly, and classical equations for energy and momentum no longer hold true. Instead, special relativity equations must be used to calculate the object's behavior.
To know more about speed visit :
https://brainly.com/question/17661499
#SPJ11
What is the amount of water produced when 8g of hydrogen is reacted with 32g of oxygen? a) 2moles b) 1mole c) 3 moles d) 0.5mole
Answers
Answer: It is an option (B)
Explanation:
Correct option is B)
The molecular masses of hydrogen, oxygen and water are 2 g/mole, 32 g/mole and 18 g/mole respectively.
2H
2
+O
2
→2H
2
O
2×2=4 g of hydrogen reacts with 1×32=32 g of oxygen to form 2×18=36 g of water.
Hence,
4
4
=1 g of hydrogen reacts with
4
32
=8 g of oxygen to form
4
36
=9 g of water.
9 grams of water can be produced when 8 g of hydrogen reacts with 8 g of oxygen.
HELP ASAP, EASY QUESTION
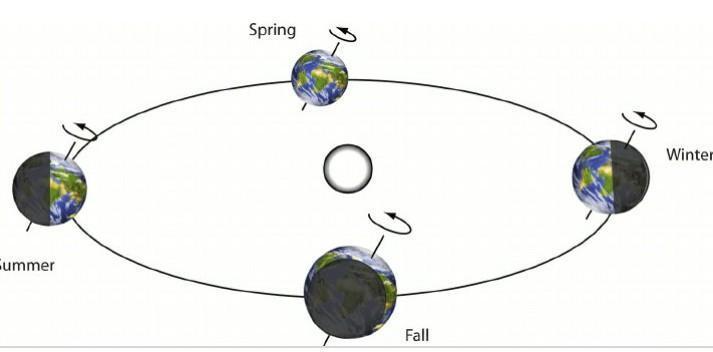
The diagram above shows the position the Earth is in in relation to the Sun during each season. You are to match the number with the correct season listed below:

Answers
Explanation:
Winter 4
Season when the northern hemisphere is tilted away from the sun.
Summer 2
Season when the northern hemisphere is tilted towards the sun.
Spring 1
Season when the northern hemisphere is neither tilted towards nor away from the sun; days getting longer and warmer.
Fall 3
Season in the northern hemisphere when the Earth is neither tilted towards nor away from the sun; days getting shorter and cooler.
You are tasked with making a series of Furosemide calibration standards for analysis by HPLC with fluorescence detection.
(a) Given Furosemide has a molecular weight of 330.7 g/mol, what weight of furosemide would you need to make a stock solution of 0.001 M (10-3 M) concentration ?
(b) How would you then make up a series of standards from this stock solution, of concentrations 10-4, 10-5, 10-6, 10-7 and 10-8 M Furosemide?
Answers
(b) To make up a series of standards from the stock solution, you would first dilute the stock solution with water to make a working solution of 10-4 M concentration. You would then take 1 mL of the working solution and dilute it with water to make a 10-5 M concentration standard, and so on, until you have standards of 10-4, 10-5, 10-6, 10-7, and 10-8 M concentrations.
What is the distance between two spheres, each with a charge of 2.4×10^-6 C, when the force between them in 0.50 N?
Answers
Answer: Use the formula F= k q1q2/r^2 where k= 1/4π€ and it's value in air is 9× 10^9.
q1=q2= 2.4×10^-6C
F= 0.5N
You will get the value of r^2 and then will have to find the squre root of that value.
Explanation:
what is the maximum distance that matter is displaced from the resting position
Answers
Amplitude is the maximum distance that matter is displaced from the resting position. Amplitude is a measure of the maximum deviation or displacement of a wave or oscillation from its average or rest position.
In physics, the amplitude is used to describe the height or depth of the peaks and valleys of a periodic wave, such as a sine wave or the oscillation of a pendulum. In this sense, the amplitude can be used to describe the maximum displacement of matter in some situations, such as the maximum angle through which a pendulum swings from its resting position.
Key points:
Amplitude is a measure of maximum deviation from the average or rest position of a wave or oscillationUsed to describe the height or depth of peaks and valleys of a periodic wave, such as a sine wave or pendulum swingCan be used to describe the maximum displacement of matter in some situations, such as the maximum angle of the pendulum swingAmplitude is only one aspect of maximum displacement and does not take into account other factors, such as mass and elasticity, force strength, or external forces.Learn more about amplitude here:
https://brainly.com/question/19036728
#SPJ4
This hydrocarbon is incomplete. Draw the hydrogen atoms and the bonds connecting them to carbon atoms such that each carbon atom has four bonds. Then record the number of hydrogen atoms you drew using a text box.

Answers
The number of the hydrogen atoms that would be required from the diagram is 10.
What is a saturated compound?A Saturated compound has all its carbon atoms connected by single bonds, and each carbon atom is bonded to the maximum number of hydrogen atoms possible. This arrangement allows the compound to have no available or unsaturated bonds for additional atoms.
The compound that is shown must be butane as such the number of the hydrogen atoms that it contains is a total of ten.
Learn more about hydrocarbon:https://brainly.com/question/32019496
#SPJ1
Which gases in the following list exist as separate atoms: hydrogen, helium, nitrogen, oxygen, neon.
Select one:
a. Hydrogen and helium
b. Helium and nitrogen
c. Helium and neon
d. Oxygen and nitrogen
Answers
helium and neon ( answer C)
Give the IUPAC name for (CH3)2C=CHCH2CH2OH. Spell out the full name of the compound. Submit Request Answer
Previous question
Answers
The IUPAC name for (CH3)2C=CHCH2CH2OH is 4-methyl-2-penten-1-ol the parent chain of the compound is a five-carbon chain, which is a pentene. The double bond is located between the second and third carbon atoms, and there is a methyl group attached to the fourth carbon.
The hydroxyl group is located at the first carbon, which gives the suffix -ol. Therefore, the name of the compound is 4-methyl-2-penten-1-ol. The numbering of the carbon atoms starts from the end closest to the double bond, which gives the smallest number to the hydroxyl group.
Learn more about compound here:
https://brainly.com/question/13516179
#SPJ11
Write 3-4 sentences to describe calorimetry and what it is used for. Also, describe the important components of the calorimeter and the terms necessary.
Answers
Calorimetry is a scientific technique used to measure the heat transfer or energy changes associated with chemical reactions or physical processes. It involves the use of a calorimeter, which is a device designed to contain and measure these energy changes. The important components of a calorimeter include a sample chamber where the reaction takes place, a thermometer to measure temperature changes, and an insulating material to minimize heat loss to the surroundings. Terms necessary for calorimetry include heat capacity (the amount of heat required to raise the temperature of the calorimeter), specific heat capacity (the amount of heat required to raise the temperature of a substance), and the principle of conservation of energy.
what does le chateliter's principle state
Answers
which of the following dietary substances inhibits uptake of non-heme iron? a. ascorbic acid
b. lactic acid
c. tea
d. MFP
Answers
Answer:
A dietary substances inhibits uptake of non-heme iron is tea.
Explanation:
Tea is a dietary substance that inhibits the uptake of non-heme iron. Tea contains compounds called tannins, which are known to bind to non-heme iron and form insoluble complexes. These complexes are difficult for the body to absorb, leading to reduced iron absorption.
On the other hand, ascorbic acid (vitamin C) enhances the absorption of non-heme iron when consumed together in the same meal. It helps convert non-heme iron into a more absorbable form, promoting its uptake by the body.
Lactic acid and MFP (meat, fish, and poultry) do not inhibit the uptake of non-heme iron. Lactic acid is produced during fermentation and is not known to interfere with iron absorption. MFP, being a source of heme iron, can actually enhance the absorption of non-heme iron due to its interaction with iron in the digestive system.
Learn more about tea here, https://brainly.com/question/16736079
#SPJ11
water (h2o) and methanol ch3oh are infinitely soluble in each other. what is the primary intermolecular force responsible for this? london dispersion forces ion - dipole interactions h- bonding
Answers
The primary intermolecular force responsible here is london dispersion forces
An intermolecular force is an attractive force that arises between the positive components or also called as protons of one molecule and the negative components or electrons of another molecule. Various physical and also chemical properties of a substance are dependent on this force.
The London dispersion force is a temporary attractive force that generally results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction as well
This type of intermolecular force of attraction is generally seen in water and methanol system.
To know more about london dispersion forces
https://brainly.com/question/20514601
#SPJ4
if the concentration of a reactant is doubled and the reaction rate is unchanged, what must be the order of the reaction?
Answers
If the concentration of a reactant is doubled and the reaction rate is unchanged, the reaction must be a zero-order reaction.
The order of a reaction represents how the rate of the reaction is affected by the concentration of the reactants. It can be determined by comparing the changes in the reaction rate with changes in the concentration of the reactants.
In a zero-order reaction, the reaction rate is independent of the concentration of the reactants. This means that doubling the concentration of a reactant will not have any effect on the reaction rate. In contrast, for a first-order reaction, doubling the concentration of a reactant would result in a doubling of the reaction rate. Similarly, for a second-order reaction, doubling the concentration of a reactant would lead to a fourfold increase in the reaction rate.
Since the given scenario states that the reaction rate remains unchanged despite doubling the concentration of the reactant, it implies that the reaction is a zero-order reaction.
Learn more about reactant: https://brainly.com/question/6421464
#SPJ11
give the systematic name for each compound. Spelling counts.
a)CIF(subscript 3)
b)CI(subscript 2)O(subscript 7)
Answers
Answer:
a) Chlorine Trifluoride
b) Dichlorine Heptoxide
GOOD LUCK FOR FUTURE! :)
I NEED HELP WITH CHEM PLEASE HAVE A DECENT ANSWER

Answers
Answer:
x is HCl because pH = 2 (acid and it reacted with Mg plus it turned litmus paper to red)
y is NaOH because pH = 10 (base and turned litmus paper to blue)
z is NaCl because pH = 7 (neutral and no reaction to litmus paper and Mg)