how do I write both conversion factors for converting L to ML
Answers
The capacity of a liquid is measured in metric units of volume called litres and millilitres.
There are four units of measurement for liquid volume: millilitres, centilitres, litres, and kiloliters.Liter is a fundamental metric unit that measures liquid volume and is equal to one cubic decimeter.The millilitre is a more compact metric unit that measures a liquid's volume or capacity. It is equivalent to one thousandth of a litre and is used to measure smaller amounts of liquid.By dividing the given amount by 1000, we may convert the given amount to millilitres. Let's, for illustration, convert 6 litres to millilitres. So, 6 × 1000 Equals 6000 ml. 6 litres hence equal 6000 millilitres.It should be remembered that we must divide the supplied amount by 1000 in order to convert from millilitres to litres. Let's convert 7000 millilitres to litres as an example. 7000 x 1000 equals 7 litres. So, 7000 millilitres equals 7 litres.A crucial component of measurement is unit conversion, which is done using the appropriate quantity conversion factor.
Learn more about unit measurement here:
https://brainly.com/question/141163
#SPJ9
Related Questions
if a reaction vessel initially contains an n2o4 concentration of 0.0550 m at 500 k , what are the equilibrium concentrations of n2o4 and no2 at 500 k ? g
Answers
The relationship between the product creation and the utilised reactant is provided by the equilibrium constant. Dinitrogen tetroxide is present in concentrations of 0.003 M and 0.054 M, respectively, of nitrogen dioxide.
The equilibrium constant is what?
The rate at which the reactant in a chemical reaction transforms into the products is expressed as an equilibrium constant.
The response can be demonstrated by,
The concentrations are estimated as follows using the ICE table that is linked below:
Continuing to solve
Consequently, nitrogen dioxide is present in concentrations of 0.054 M and 0.003 M, respectively.
Here is more information about the equilibrium constant :
brainly.com/question/26807607
#SPJ4
What causes the sea floor to move apart at a sea floor spreading center A density B continental drift C paleomagnetism D convection currents
Answers
Answer: D convection currents
Explanation:
The seafloor spreading is a phenomena that occurs due to liberation of heat from the convection currents generated in the mantle. It makes the earth crust more plastic and less dense. This happens at divergent plate boundaries. As the plates move apart, the less denser material rises. It leads to the formation of mountain and crust cracks.
Boron has an average atomic mass of 10.81. One isotope of boron has a mass of 10.012938 and a relative abundance of 19.80 percent. The other isotope has a relative abundance of 80.20 percent.
What is the mass of that isotope? Report to two decimal places.
amu
Answers
Answer:
11.01
Explanation:
because yes :)
Which material is the limiting ingredient if you have 2 cans of tomatoes, 2 cups green beans, 2 cups pasta, 8 cans of beans, and 12 cups broth
2 cans diced tomatoes
1 cup green beans
1/2 cup pasta
1 can beans
4 cups broth
Answers
Based on the recipe for preparing the food, the limiting ingredient is the 2 cans of tomatoes.
What is the recipe for the food?The recipe for the food is given below:
2 cans diced tomatoes1 cup green beans1/2 cup pasta1 can beans4 cups brothHence, 2 cans of diced tomatoes require 1 cup of green beans, 1/2 cup of pasta, 1 can of beans, and 4 of cups broth.
Hence, after, the 2 cans of diced tomatoes are used up, no more food can be prepared.
A limiting reagent or limiting ingredient is used up when cooking, hence, the 2 cans of tomatoes are the limiting ingredient.
Learn more about limiting reagent at; https://brainly.com/question/23661051
#SPJ1
what are the difference between hemogeneous mixture and heterogeneous mixture ??
Answers
Answer:
Refer to attached file below
Hope it helps..
Have a great day :P

why do atoms become anions and cations
Answers
Answer:
Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. ... And all of them form an anion with a single negative charge. The VIA elements gain two electrons to form anions with a 2- charge.
Explanation: OR
Metallic atoms hold some of their electrons relatively loosely. Consequently, they tend to lose electrons and form cations. Conversely, most nonmetallic atoms attract electrons more strongly than metallic atoms, and so gain electrons to form anions.
Metallic atoms hold some of their electrons relatively loosely. Consequently, they tend to lose electrons and form cations. Conversely, most nonmetallic atoms attract electrons more strongly than metallic atoms, and so gain › cat...
_
Answer:
It gains one or more electrons from another atom to become negatively charged.
Explanation:
An atom becomes charged by either gaining or losing electrons, and is called an ion. An atom with less than the normal number of electrons is a positive ion (a cation), and an atom with one or more extra electrons is a negative ion (an anion).
A colorimeter is an instrument used to measure the amount of Choose... absorbed by a solution. This absorbance is proportional to the Choose... of the solute in soli Theat light sound absorbed by a solution. This absorbance is A colorimeter is an instrument used to measure the amount of Choose... - proportional to the Choose... of the solute in solution. concentration purity molecular weight
Answers
A colorimeter is a device used to gauge how much heat a solution has absorbed. The concentration of the solute in the solution is inversely correlated with this absorbance.
What is a calorimeter?
A calorimeter is a tool used in calorimetry, a technique for calculating heat capacity and measuring the heat of chemical processes or other physical changes. Among the most popular kinds are differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters, and accelerated rate calorimeters.
One at a time, two chemicals A and B are placed in a calorimeter, and the temperatures before and after the reaction are recorded. The enthalpy change per mole of substance A in the process is calculated using these data. The mass and specific heat capacity of the components can be multiplied by the temperature change to provide an estimate of the amount of energy produced or absorbed during the reaction. The enthalpy change of the reaction is calculated by dividing the energy change by the quantity of A that was present.
One at a time, two chemicals A and B are placed in a calorimeter, and the temperatures before and after the reaction are recorded. The enthalpy change per mole of substance A in the process is calculated using these data. The mass and specific heat capacity of the components can be multiplied by the temperature change to provide an estimate of the amount of energy produced or absorbed during the reaction. The enthalpy change of the reaction is calculated by dividing the energy change by the quantity of A that was present.
to learn more about Colorimeter click the link below
brainly.com/question/29384804
#SPJ4
Help me please I’ll give brainliest answer

Answers
Answer:
the answer is c I believe
A gas expands from a volume of 3.0 dm3 to 5.0 dm3 against a constant pressure of 3.0 atm. The work done during expansion is used to heat 10.0 mole of water of temperature 290.0K. Calculate the final temperature of water (specific heat of water =4.184 J K−1g−1)
Answers
the final temperature of water comes out to be 290.877 K. The quantity of work completed during the expansion must be determined in order to calculate the energy supplied to the water and the water's final temperature.
Following the gas expansion, we can apply the following equation to determine the water's final temperature:
q = mcΔT
Where: q = the heat the water absorbs
m = the water's mass
c is the water's specific heat capacity.
T stands for temperature change.
Let's start by calculating the heat that the water absorbed during the gas expansion:
q = the work that the gas does
The equation: can be used to determine how much work the gas is doing.
w = -PΔV
Where: w = job completed
Pressure is P.
V stands for volume change
We can determine the work done if we know that the pressure (P) is 3.0 atm and the change in volume (V) is 5.0 dm3 - 3.0 dm3 = 2.0 dm3.
w = 3.0 atm x 2.0 dm3, which is -6.0 atm dm3.
The heat absorbed by the water will be positive since the work completed, which represents work on the system, is negative:
Q=-w=6.0 atm dm3
Next, we must convert the work done's units to joules:
1 atm dm3 equals 101.375 J
At STP, 1 mol of gas takes up 22.4 dm3.
6.0 atm dm3 multiplied by 101.325 J/atm dm3 results in 607.95 J.
Now, we can determine the water's temperature change (T):
q = mcΔT
10 mol * 18.015 g/mol * 4.184 J/g K * 10.795 J = 607.95 J ΔT
753.78 g * 4.184 J/g K * T = 607.95 J
T = 753.78 g * 4.184 J/g K / 607.95 J
ΔT ≈ 0.180 K
The ultimate temperature is then determined by adding the temperature change to the 290.0 K starting point:
Final temperature = 290.0 K plus 0.180 K, or 290.180 K.
to know more about specific heat refer to the link below
https://brainly.com/question/27862577
#SPJ4
This atom can form up to _____ single covalent bond(s). An atom with two electron shells. There is one electron pair in the inner electron shell and four unpaired electrons in the outer electron shell. 0 1 4 2 3
Answers
Answer:
4
Explanation: it's a carbon atom right....soo it'll form 4 bonds(single)
An atom can form 4 single covalent bonds is carbon with two electron shell.
What are covalent bonds?The bonds are defined as a strong bond that binds atoms, ions, or molecules together and promotes the production of molecules.
There are three types of bonds.
Ionic bondCovalent bondMetallic bondCovalent bonds are defined as the bond formed by sharing of electrons with each other.
Covalent bonding is a stable equilibrium of the attractive and repulsive forces between two atoms that occurs when they share electrons. Bonding pairs or sharing pairs are other names for these electron pairs.
It can also be defined as a type of chemical link where atoms share electrons to create electron pairs.
Covalent bonds has low melting point, boiling point, enthalpy of fusion and enthalpy of vaporization.
Thus, an atom can form 4 single covalent bonds is carbon with two electron shell.
To learn more about covalent bonds, refer to the link below:
https://brainly.com/question/10777799
#SPJ12
PLZ HELP I WILL GIVE YOU 30 POINTS
You are designing an experiment to test the efficiency of different bikes. What simple machines helped to make this complex machine?
wedge, pulley, and wheel and axle
wedge, screw and wheel and axle, inclined plane
screw, inclined plane and wheel and axle, pulley
screw, lever, and wheel and axle
Answers
Answer:
C
Explanation:
you need a wheel And axle for the bike.
You need the pulley for the chain to pull the tires
you need the inclined plane to test the bike
you need a screw to make sure the wheel and axle is in place.
Answer:
C
Explanation:
im doing the test
which statement(s) regarding metal ion indicators in edta titrations is/are true? metal ion indicators are compounds that change color when they bind to a metal ion. useful indicators must bind the metal ion less strongly than edta does. metal ion indicators are also acid-base indicators.
Answers
Metal ion indicators are compounds that change color when they bind to a metal ion. They are commonly used in EDTA (ethylene diamine tetraacetic acid) titrations, where EDTA acts as a chelating agent, binding to the metal ion to form a stable complex.
One important characteristic of a useful metal ion indicator is that it must bind the metal ion less strongly than EDTA does. This is because EDTA is typically added in excess to ensure complete complexation of the metal ion, and a strong metal ion indicator would compete with EDTA for the metal ion and interfere with the accuracy of the titration.
Metal ion indicators are not necessarily also acid-base indicators, but some can be both. In EDTA titrations, pH plays an important role in determining the stability of the metal-EDTA complex, so an indicator that can monitor pH changes as well as metal ion binding can be useful.
In summary, metal ion indicators are compounds that change color when they bind to a metal ion, and useful indicators must bind the metal ion less strongly than EDTA does.
To learn more about titrations refer to:
brainly.com/question/2728613
#SPJ4
How can you show using Pauli's exclusion principle that p sub shell can have only 6 electrons?

Answers
where l = subshell value.
"l"values of subshell are.
s = 0.
p = 1.
d = 2.
f = 3.
So in p orbital we have 6 electrons.
8. What is a quasar?
Answers
Compare and contrast iron and neon
Answers
Answer:
neon is a gas.it has full of sub shell.but iron hasnt full of sub shell so iron is react with other elements
What’s the density in g/cm^3

Answers
Answer:
0.0027 gram/cubic centimeter
cc=ml
Explanation:
pls vote brainliest ❤❤
What does the absorption spectrum of an atom show?
Answers
The absorption spectrum of an atom shows pattern of dark lines or bands attained by transmitting absorbed light beam into the spectroscope.
What does an atom's absorption spectrum look like?By sending an absorbed light beam into a spectroscope, one can create a pattern of black lines or bands.
The vapors or solution of a substance in the discharge tube are illuminated by a white light. After passing through a spectroscope, the transmitted light produces a spectrum of black lines at specific wavelengths. These shady lines match the light radiation wavelengths that the material has absorbed.
An atom, ion, or molecule absorbs photons with energies equal to the energy difference between the two states when it transitions from one to the other. An absorbance spectrum that depicts the intensity of emission as a function of wavelength is the end result.
Learn more about atom at:
https://brainly.com/question/6258301
#SPJ1
Learn more about Ascribed status at:
brainly.com/question/1266835
#SPJ1
what are the similarities shared between solids and gases?
Answers
A researcher titrates a 500 mL solution of 2 M C2H5OCOOH (lactic acid, structure shown below) with a 1 M KOH solution. What is the pH at the equivalence point at 25°C?
Ka of lactic acid = 1.4 x 10–4
A. 8.8 B. 10.1 C. 9.1 D. 3.9 E. 12.1
Answers
The pH at the equivalence point at 25°C is 8.8. The pH at the equivalence point can be calculated using the following equation: pH = p Ka + log([salt]/[acid])Where salt is the potassium lactate and acid is the lactic acid.
Correct option is , A. 8.8.
A reaction occurs when a strong base, such as potassium hydroxide, KOH, is combined with a weak acid, such as lactic acid, C2H5OCOOH. The weak acid is initially present in excess, and the pH is calculated using the Henderson-Hasselbalch equation at the start of the titration. pH = pKa + log([A-]/[HA])The weak acid and its conjugate base are present in equal concentrations at the equivalence point. Because the pH is a function of the ratio of acid and base concentrations, the pH of a weak acid solution equals its pKa at the equivalence point, where pKa is the acid dissociation constant.
Ka for lactic acid is 1.4 × 10−4.Using the equation, we get:pH = pKa + log([salt]/[acid]) = 3.85 + log([K+][lactate−]/[lactic acid])At the equivalence point, the total volume of the solution is 1 L (500 mL of 2 M C2H5OCOOH solution is used, which is equivalent to 1 mol of acid).Since it reacts with 1 mol of KOH, which is equivalent to 1 mol of potassium lactate, the concentration of potassium lactate is 1 M and the concentration of lactic acid is 0.5 M.At 25°C.
To know more about potassium visit:
https://brainly.com/question/13321031
#SPJ11
Which substance is readily soluble in hexane (C6H14)?
A. H2O
B. PCl3
C. KOH
D. C3H8
Answers
The substance that is readily soluble in hexane (C₆H₁₄) is D. C₃H₈. Hence, option D is correct.
Hexane is a nonpolar solvent, and substances that are nonpolar or have similar nonpolar characteristics tend to be soluble in hexane. Among the given options, C₃H₈ (propane) is a nonpolar hydrocarbon and is, therefore, readily soluble in hexane.
A. H₂O (water) is a polar molecule and is not soluble in hexane.
B. PCl₃ (phosphorus trichloride) is a polar molecule and is not soluble in hexane.
C. KOH (potassium hydroxide) is an ionic compound and is not soluble in hexane.
Thus, the correct answer is D. C₃H₈. Hence, option D is correct.
Learn more about solubility from the link given below.
https://brainly.com/question/31493083
#SPJ4
floridium atoms (a hypothetical metal) are in a face-centered cubic unit cell, and the edge length of the unit cell is 310.2 pm. what is the atomic radius of floridium in pm to one decimal place?
Answers
The atomic radius of Floridium atoms in the FCC unit cell is approximately 109.8 pm to one decimal place.
The face-centered cubic unit cell contains 4 atoms. The edge length of the unit cell can be calculated using the formula:
a = 2 * (radius of atom)
Therefore, the radius of each floridium atom can be calculated as:
radius of atom = a / (2 * sqrt(2))
radius of atom = 310.2 pm / (2 * sqrt(2))
radius of atom = 109.9 pm
Therefore, the atomic radius of floridium is 109.9 pm to one decimal place.
To find the atomic radius of Floridium atoms in a face-centered cubic (FCC) unit cell, we can follow these steps:
1. Recall the relationship between the edge length (a) and the atomic radius (r) in an FCC unit cell: a = √2 * 4r.
2. Solve for the atomic radius (r) using the given edge length (a = 310.2 pm).
Step 1: Relationship between edge length and atomic radius in an FCC unit cell
For an FCC unit cell, the relationship between the edge length (a) and the atomic radius (r) is given by the formula:
a = √2 * 4r
Step 2: Solve for the atomic radius (r)
We are given the edge length (a = 310.2 pm). We can plug this value into the formula and solve for r:
310.2 pm = √2 * 4r
Now, we need to isolate r by dividing both sides of the equation by 4√2:
r = (310.2 pm) / (4√2)
r ≈ 109.8 pm
Therefore, the atomic radius of Floridium atoms in the FCC unit cell is approximately 109.8 pm to one decimal place.
learn more about atoms here
https://brainly.com/question/14352690
#SPJ11
In the electrolysis of molten lead bromide, what is the product at the anode?

Answers
Answer:
it is the second one. Lead
Which of these metals alloys would be the strongest?
By strongest, meaning most difficult to damage or change shape through force.
a Fe-Ni-C (Steel alloy of iron)
b W2C
c Solid carbon - Graphite
d Fe(s)
Answers
According to the forces of attraction the tungsten metal alloy that is W₂C( tungsten carbide) is most difficult to damage or change shape through force.
What are forces of attraction?Forces of attraction is a force by which atoms in a molecule combine. it is basically an attractive force in nature. It can act between an ion and an atom as well.It varies for different states of matter that is solids, liquids and gases.
The forces of attraction are maximum in solids as the molecules present in solid are tightly held while it is minimum in gases as the molecules are far apart . The forces of attraction in liquids is intermediate of solids and gases.
The physical properties such as melting point, boiling point, density are all dependent on forces of attraction which exists in the substances.
Learn more about forces of attraction,here:
https://brainly.com/question/10957144
#SPJ1
How many moles of KBr will be produced from 7 moles of BaBr2?
BaBr2 + K2SO4 --> 2 KBr + BaSO4
1 mole KBr
7 moles KBr
3.5 moles KBr
14 moles KBr
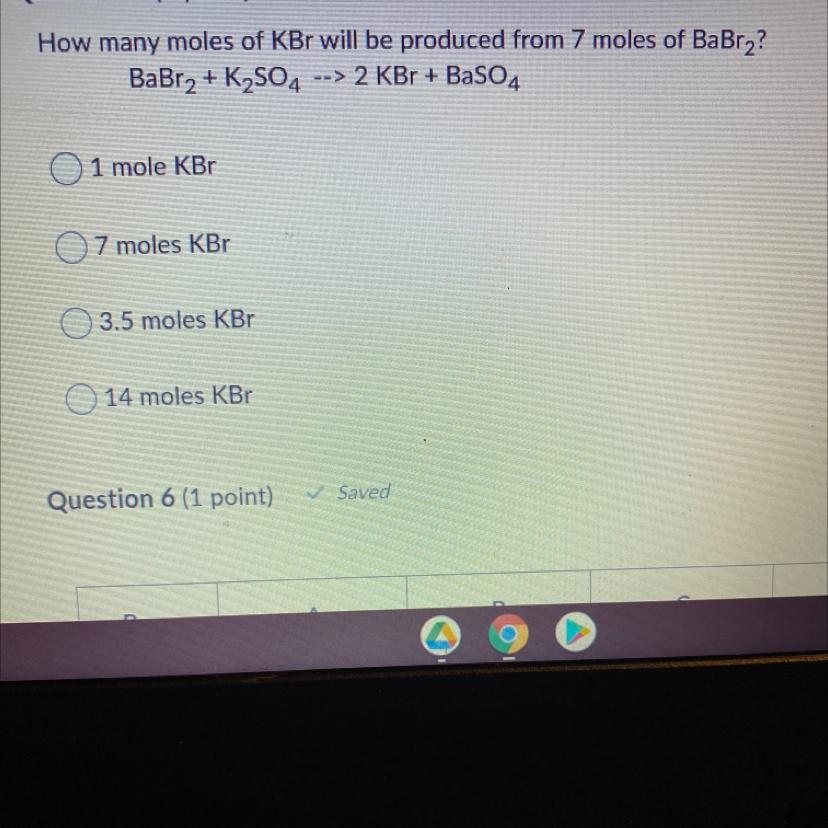
Answers
1 mol BaBR2 produces 2mol KBr .
7 mol s of BaBr_2 produces:-
7(2)14mol KBrOption D is correct
What volume of oxygen at 455 K and a pressure of 127400 Pa is produced by the decomposition of 114.7 g of BaO2 to BaO and O2?
Answers
Answer:
10 L
Explanation:
The equation of the reaction is;
2BaO2 = 2BaO + O2
Number of moles of BaO2 = 114.7 g/169.33 g/mol = 0.677 moles
From the reaction equation;
2 moles of BaO2 yields 1 moles of O2
0.677 moles of BaO2 yields 0.677 * 1/2 = 0.3385 moles of oxygen
Hence;
PV=nRT
V = ?
P = 127400 Pa or 1.257 atm
T = 455 K
n = 0.3385 moles
R = 0.082 atmLmol-1K-1
V = nRT/P
V = 0.3385 * 0.082 * 455/1.257
V= 10 L
A. An element with the valence electron configuration 4s2 would form a monatomic ion with a charge of ____. In order to form this ion, the element will (lose/gain) (#) electron(s) from/into the ____ subshell(s).
B. An element with the valence electron configuration 2s^2 2p^4 would form a monatomic ion with a charge of ____. In order to form this ion, the element will (lose/gain) # electron(s) from/into the ____ subshell(s).
Answers
Answer:
A. 4s subshell.
B. An element with the valence electron configuration 2s² 2p⁵ would form a monatomic ion with a charge of -1.
In order to form this ion, the element will gain 1 electron into the 2p subshell
A) The element with 4s² configuration form a monatomic ion with a charge of +2 and the element will lose electrons from the 4s subshell.
B) The element with 2s²2p⁴ configuration form a monatomic ion with a charge of -2 and the element will gain electrons into the 2p subshell.
What is a valence electron?Valence electrons are the electrons occupying the outermost shell of an atom while the electrons in the inner shell are called core electrons. Lewis structures are helpful to determine the number of valence electrons and knowing the types of bonds.
Valence electrons are filled in different shells and these electrons are caused interaction between atoms and lead to the formation of chemical bonds.
Only electrons present in the outermost shell can contribute to the formation of a chemical bond or a molecule and decide the reactivity of the element.
The element with a 4s² configuration forms a monatomic ion as it loses both electrons present in the 4s subshell. This will lead to a charge of +2 on the element and the element from a monoatomic ion with +2 charge.
The element with 2s²2p⁴ configuration will accept two electrons into the 2p subshell. So that it can get the nearest noble gas configuration and it will form a monoatomic ion with -2 charge.
Learn more about valence electrons, here:
brainly.com/question/18612412
#SPJ2
which of these liquids is the least acidic?

Answers
Answer:
The answer is lemonade.
Weaker acids are those which tend to have a higher pH from 4 to at least 6 stronger acids have low pH from 2 and below that's from 2 to 0.
Hope this helps
Hope
how many significant figures are in 120 miles?
Answers
1 and 2 are the significant zeros.
pls mark brainliest
What causes elements to react to each other
Answers
Answer:
One atom of each element is made up of protons, neutrons, and electrons. The number of electrons determines how an element reacts. The number of protons gives the element its identity. ... They react well with nonmetals because they can easily give up electrons to form ions.
A saturated solution of Ca(OH)₂ has a pH of 12.40. What is the Ksp of Ca(OH)₂? Calculate the maximum pH that could be achieved in a solution of manganese (II) hydroxide, Mn(OH)₂ (Ksp = 2.1 times 10⁻¹³).
Answers
The Ksp of Ca(OH)₂ is approximately 1.58 × 10 ⁻⁵
The maximum pH achievable in a solution of Mn(OH)₂ (Ksp = 2.1 × 10 ⁻¹³ is approximately 7.16.
How to calculate Ksp of Ca(OH)₂?To determine the Ksp (solubility product constant) of Ca(OH)₂, we can use the pH of the saturated solution. The pH of 12.40 indicates the concentration of hydroxide ions (OH⁻) in the solution.
pOH = 14 - pH
pOH = 14 - 12.40
pOH = 1.60
Since Ca(OH)₂ dissociates into two OH⁻ ions for every formula unit, the concentration of OH⁻ ions is twice the concentration of Ca(OH)₂.
[OH⁻] = 2 * 10^(-pOH)
[OH⁻] = 2 * 10^(-1.60)
[OH⁻] = 0.0251 M
The concentration of Ca²⁺ ions is the same as the concentration of OH⁻ ions since the compound is 1:1.
[Ca²⁺] = [OH⁻] = 0.0251 M
Now we can calculate the Ksp of Ca(OH)₂ using the concentrations:
Ksp = [Ca²⁺] * [OH⁻]²
Ksp = (0.0251) * (0.0251)²
Ksp ≈ 1.58 × 10 ⁻⁵
Therefore, the Ksp of Ca(OH)₂ is approximately 1.58 × 10 ⁻⁵
Now let's calculate the maximum pH that could be achieved in a solution of Mn(OH)₂ with a Ksp of 2.1 × 10 ⁻¹³
Since Mn(OH)₂ dissociates into two OH⁻ ions for every formula unit, we need to calculate the concentration of OH⁻ ions at equilibrium using the Ksp.
Ksp = [Mn²⁺] * [OH⁻]²
2.1 × 10 ⁻¹³ = [OH⁻]²
[OH⁻] = sqrt(2.1 × 10 ⁻¹³
[OH⁻] ≈ 1.45 × 10⁻⁷M
To calculate the pH, we can use the pOH equation:
pOH = -log[OH⁻]
pOH = -log(1.45 × 10⁻⁷
pOH ≈ 6.84
since pH + pOH = 14, we can determine the maximum pH:
pH = 14 - pOH
pH = 14 - 6.84
pH ≈ 7.16
Therefore, the maximum pH that could be achieved in a solution of manganese (II) hydroxide, Mn(OH)₂, with a Ksp of 2.1 × 10 ⁻¹³ ), is approximately 7.16.
Learn more about: Ksp (solubility product constant)
brainly.com/question/17101393
#SPJ11