How do human wants and needs affect the products that are used and created?
Answers
Answer:
They are the products you can purchase to meet your wants and needs. ... Individuals no longer create most of the products and services they consume as ... Economic resources are the things available to be used to produce goods and services. ... resources are natural resources, human resources, and capital resources.
Related Questions
What is the concentration of CN- ion in a 0.100 molar solution of K4Fe(CN)6? Kd for(Fe(CN)6)4- is 1.3 × 10^-37.1. 8.33 × 10^-7 M2. 3.87 × 10^-6 M3. 5.00 × 10^-6 M4. 2.32 × 10^-5 M5. 2.23 × 10^7 M
Answers
The concentration of CN⁻ ions in the 0.100 M solution of K₄Fe(CN)₆ is 3.87 × 10⁻⁶ M . Option 2 is Correct.
To find the concentration of CN- ions in a 0.100 M solution of K₄Fe(CN)₆, we first need to consider the dissociation reaction:
Fe(CN)₆⁴⁻ → Fe³⁺ + 6CN⁻
Since Kd for (Fe(CN)₆⁴⁻) is 1.3 × 10⁻³⁷, we can set up the following expression:
\(Kd=\frac{[H+][A-]}{[HA]}\)
The energy needed to produce a complex ion at a specific ion concentration is measured by the equilibrium constant, or Kd. This implies that complexation processes can lead
Kd = [Fe³⁺][CN⁻]⁶ / [Fe(CN)₆⁴⁻]
Assuming the reaction proceeds to completion, we have:
0.100 M Fe(CN)₆⁴⁻ → 0.100 M Fe³⁺ + 0.600 M CN⁻
Now, we can substitute these values into the Kd expression:
1.3 × 10⁻³⁷ = (0.100)(0.600)⁶ / (0.100)
Solving for the concentration of CN⁻ ions:
[CN⁻] = 3.87 × 10⁻⁶ M
Learn more about concentration here
https://brainly.com/question/24660426
#SPJ11
The Complete question is
What is the concentration of CN- ion in a 0.100 molar solution of K₄Fe(CN)₆? Kd forFe(CN)₆ - is 1.3 × 10⁻³⁷.
1. 8.33 × 10⁻⁷ M
2 . 3.87 × 10⁻⁶ M
3. 5.00 × 10⁻⁶ M
4. 2.32 × 10⁻⁵ M
5. 2.23 × 10⁷ M
What do sex hormones do?
Answers
Answer:
Hormones help regulate many bodily processes, such as appetite, sleep, and growth. Sex hormones are those that play an essential role in sexual development and reproduction. The main glands that produce sex hormones are the adrenal glands and the gonads, which include the ovaries in females and testes in males
Explanation:
The following balanced equation shows the formation of ethane (C2H6). C2H2 2H2 mc022-1. Jpg C2H6 How many moles of hydrogen are needed to produce 13. 78 mol of ethane? 3. 445 mol 6. 890 mol 27. 56 mol 55. 12 mol.
Answers
The moles of hydrogen required for the synthesis of 13.78 mol ethane has been 27.56 mol. Thus, option C is correct.
The balanced chemical equation for the synthesis of ethane has been:
\(\rm C_2H_2\;+\;2\;H_2\;\rightarrow\;C_2H_6\)
In a balanced chemical equation for the synthesis of 1 mole of ethane, 2 moles of hydrogen is required.
The moles of hydrogen required for the synthesis of 13.78 mol of ethane has been:
\(\rm 1\;mol\;C_2H_6=2\;mol\;H_2\\13.78\;mol\;C_2H_6=13.78\;\times\;2\;mol\;H_2\\13.78\;mol\;C_2H_6=27.56\;mol\;H_2\)
The moles of hydrogen required for the synthesis of 13.78 mol ethane has been 27.56 mol. Thus, option C is correct.
For more information about balanced equation, refer to the link:
https://brainly.com/question/7181548
Which substance is an electrolyte?
A)
C2H5OH(C)
B)
NaOH(s)
C)
H2(g)
D)
C6H1206 (s)
Answers
Answer:
B)NaOH(s)
Explanation:
a new type of porous material serves as food packaging film, as shown in the figure below. the film rests on a nonporous metal plate. the packaging film is 0.20 cm thick (2.0 mm). the food contains liquid water, which exerts a vapor pressure of 0.030 atm at 25 c. at 25 c, the effective diffusion coefficient of water vapor in the porous film is 1.3 10 4 cm2/s, and the molecular diffusion coefficient of water vapor in air is 0.26 cm2/s. you may assume that the total system pressure is constant at 1.0 atm, and initially, the gas space of the porous film contains air and no water vapor. how long will it take for the water vapor to achieve a partial pressure of 0.0150 atm at the back surface of 27.9 the film (z 0) at 25 c?
Answers
The problem requires calculating the time required for water vapor to achieve a partial pressure of 0.0150 atm on the back surface of a new type of porous material used as food packaging film.
The film is 0.20 cm thick, rests on a nonporous metal plate, and contains liquid water with a vapor pressure of 0.030 atm at 25°C. The effective diffusion coefficient of water vapor in the porous film is 1.3 10 4 cm2/s, and the molecular diffusion coefficient of water vapor in air is 0.26 cm2/s. Assuming that the total system pressure is constant at 1.0 atm and the gas space in the porous film initially contains air, it will take approximately 1100 seconds or 18.3 minutes for the water vapor to reach the desired partial pressure at the back surface of the film.
To determine the time required for the water vapor to achieve a partial pressure of 0.0150 atm at the back surface of the porous film, we need to use Fick's first law of diffusion: J = -D(dC/dz), where J is the diffusion flux, D is the effective diffusion coefficient (1.3 x 10^-4 cm^2/s), and dC/dz is the concentration gradient.
The concentration gradient can be found using the vapor pressure difference (0.030 - 0.0150 atm) and the film thickness (0.20 cm). Convert the pressure difference to concentration using the Ideal Gas Law: C = P/RT, where R is the gas constant (82.06 cm^3.atm/mol.K) and T is the temperature (25°C or 298 K).
Once the concentration gradient is found, solve for the diffusion flux J using Fick's first law. Then, divide the initial concentration of water vapor by the diffusion flux to find the time required for the water vapor to achieve a partial pressure of 0.0150 atm at the back surface of the film.
To know about pressure :
https://brainly.com/question/30673967
#SPJ11
6. Find the density of the cube in #3 if it has a mass of 100 g.
Answers
Answer:
the density of the cube is 0.8 grams per cubic centimeter.
Explanation:
To find the density of the cube, we need to know its volume as well as its mass. From problem #3, we know that the length of each side of the cube is 5 cm. Therefore, the volume of the cube is:
Volume = (side length)^3
Volume = 5^3 cm^3
Volume = 125 cm^3
Now that we know the volume of the cube, we can use the formula for density:
Density = Mass / Volume
We are given that the mass of the cube is 100 g. Substituting the values we get:
Density = 100 g / 125 cm^3
Simplifying, we get:
Density = 0.8 g/cm^3
Therefore, the density of the cube is 0.8 grams per cubic centimeter.
how many significant figures are in 120 miles?
Answers
1 and 2 are the significant zeros.
pls mark brainliest
What happens when Mg forms an ionic bond?
o it gains one electron and becomes a -1 anion
o it loses one electron and becomes a +1 cation
o It loses 2 electrons and becomes a +2 cation
It shares electrons with the other atom in the bond
Look at picture
ASAP please will mark as brainlist don’t got much time

Answers
Answer:
it gains one electron and become a -2 anion...
it is the right answer..
above answer is right explanation
A gas sample was produced in the laboratory. The gas was determined to be more dense than air (which is mostly composed of nitrogen). What is the identification of the gas? a)Hydrogen b)Neon c)Methane (CH_4) d)Carbon Dioxide
Answers
The correct option is (d) Carbon Dioxide.
Explanation:
The density of air is around 1.2 g/L, which means that any gas with a density above this value is more dense than air.
Carbon dioxide has a density of approximately 1.98 g/L, which is considerably more dense than air (composed of nitrogen and oxygen).
As a result, if a gas sample is determined to be more dense than air, it is likely to be carbon dioxide (CO2), which has a molecular weight of 44 g/mol.
Carbon dioxide is produced in the laboratory by many chemical reactions and is commonly employed in the food and beverage industries, such as carbonating soda and beer.
To know more about chemical reactions visit;
https://brainly.com/question/29762834
#SPJ11
What is the momentum of a stationary truck with a mass of 2000 kg? Need help ASAP
Answers
Answer:
0 its stationary it has no momentum
Explanation:
describe what happens when a polar covalent and a nonpolar covalent substance are combined
Answers
The specific outcome of combining a polar covalent and a nonpolar covalent substance depends on their chemical properties, intermolecular forces, and the presence of any other substances or conditions that can influence their interaction.
When a polar covalent substance and a nonpolar covalent substance are combined, several possible outcomes can occur depending on the nature of the substances and the conditions of the combination.No reaction: If the polar and nonpolar substances are not chemically reactive with each other, they may simply coexist without any noticeable interaction.Separation: If the polar and nonpolar substances are immiscible, they may separate into distinct phases or layers. This occurs because polar substances tend to be attracted to other polar substances and repel nonpolar substances, while nonpolar substances tend to be attracted to other nonpolar substances and repel polar substances.
Limited interaction: In some cases, there may be limited interaction between the polar and nonpolar substances. This can happen when weak intermolecular forces, such as London dispersion forces, are present. These weak forces can induce temporary dipoles in the nonpolar substance, allowing for some degree of interaction with the polar substance.Emulsion or dispersion: Under certain conditions, it is possible to create an emulsion or dispersion where small droplets or particles of the nonpolar substance are dispersed within the polar substance. This occurs when an emulsifying agent or surfactant is added to stabilize the mixture.The specific outcome of combining a polar covalent and a nonpolar covalent substance depends on their chemical properties, intermolecular forces, and the presence of any other substances or conditions that can influence their interaction.
for more such questions polar
https://brainly.com/question/17118815
#SPJ11
Which of these would you MOST likely find in a country with an unlimited government?
A checks and balanceschecks and balances
B one person ruleone person rule
C protection of free speechprotection of free speech
D separation of powers
Answers
Explanation: I got it right
A greenhouse is filled with air that cotains more carbon dixoide than normal air has. How might phothsyphness and plant growth be affeacted
Answers
Answer:
See explanation
Explanation:
We know that photosynthesis involves the combination of carbon dioxide and water in the presence of sunlight to yield glucose.
If the atmosphere is rich in carbon dioxide such as in a green house where air is filled with carbon dioxide, the rate of photosynthesis is increased.
As the rate of photosynthesis is increased, the growth of plants is also increased.
Hence, in a greenhouse where the air contains more carbon dioxide, the rate of plant growth increases.
What are the coefficients when this equation is balanced explain
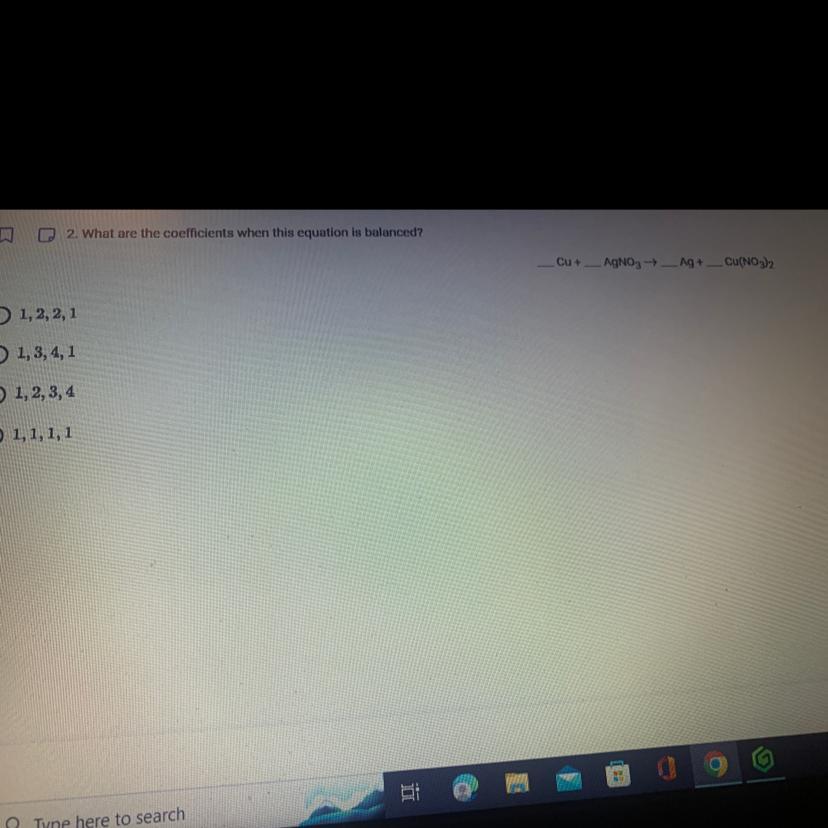
Answers
The balanced equation will be:
Cu + 2 AgNO3 ----> 2Ag + Cu(NO3)2
The balancing of equation is done to satisfy the law of conservation of mass. This law states - "mass can neither be created, nor be destroyed". Thus, the mass of the element in the equation cannot be created or destroyed, it is just shifted.
The answer is 1,2,2,1; the first option.
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity?
A. carbon
B. fluorine
C. nitrogen
D. oxygen
Answers
why doesn’t solid sodium chloride conduct electricity, but solid aluminum does?
Answers
Solid sodium chloride (NaCl) does not conduct electricity because it is an ionic compound, meaning it consists of charged ions held together by strong electrostatic forces. In its solid state, the ions are fixed in place and cannot move freely, preventing the flow of electric current.
On the other hand, solid aluminum (Al) conducts electricity because it is a metallic element. In metals, the atoms are arranged in a closely packed lattice structure, with electrons moving freely between them. These electrons, known as "delocalized electrons," are responsible for the excellent conductivity of metals like aluminum.
TO KNOW MORE ABOUT (NaCl) PROPERTY CLICK THIS LINK-
brainly.com/question/24697497
#SPJ11
What characteristics are similar between two biomes
Answers
Answer:
Niches
Explanation:
write the application of thermodynamic
Answers
Answer:
One of the most important things we can do with heat is to use it to do work for us. A heat engine does exactly this—it makes use of the properties of thermodynamics to transform heat into work. Gasoline and diesel engines, jet engines, and steam turbines that generate electricity are all examples of heat engines.
the distribution of electrons among orbitals in a many electron atom is known as its electron
Answers
The distribution of electrons among orbitals in a many-electron atom is known as its electron configuration.
The electron configuration describes how electrons are distributed in the various energy levels and orbitals around the nucleus of an atom. It is represented using a series of numbers and letters that indicate the principal energy level (n), the type of orbital (s, p, d, f), and the number of electrons in each orbital.
For example, the electron configuration for carbon (C) is 1s^2 2s^2 2p^2, which shows that carbon has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital.
The electron configuration provides important information about the arrangement and organization of electrons within an atom, which in turn affects the atom's chemical properties and reactivity.
To learn more about electron configuration, visit:
https://brainly.com/question/29184975
#SPJ11
Guyton de Morveau, a French chemist, created a system for naming compounds that is still used today. For example, he said that a compound of zinc and chorine is called zinc chloride. Which of the
following is true about de Morveau's naming system?
A. The non-metallic atom is last.
B. The metallic atom is last.
C. The larger atom is first.
D. The smaller atom is first.
Answers
In de Morceau's nomenclature scheme, the smaller atom appears first.
What naming scheme is used?Nomenclature is an organism name system used in biological classification. Genus and species names, two Latinized nouns drawn from numerous sources, serve as indicators of the species to which the creature belongs.
Who created the current nomenclature for chemical substances?On August 26, 1743, Antoine-Laurent Lavoisier was born in Paris, France. He was a well-known French chemist and a key player in the 18th century chemical revolution. In addition to co-creating the current system for identifying chemical compounds, he established an experiment-based explanation of the chemical reactivity of oxygen.
To know more about nomenclature visit:-
https://brainly.com/question/25845195
#SPJ1
Sam did an experiment. He boiled water in a teapot and saw vapor coming out. When he placed a cold plate in contact with the vapor it formed droplets of water.? Can you imagine what was that process and Why did he observe this?
Answers
Answer:
evaporation and condesation
Explanation:
because he was curiuos
for the previous light of 671 nm, if a light emitted 0.50 moles of this photon, what is the energy of this light?
Answers
The energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.92 * 10^4 Joules.
Let's understand this in detail:
To find the energy of light emitted by 0.50 moles of photons with a wavelength of 671 nm, we can follow these steps:
1. Convert the wavelength to meters: 671 nm * (1 meter / 1,000,000,000 nm) = 6.71 * 10^-7 meters.
2. Calculate the energy of one photon using the Planck's equation: E = hf, where E is energy, h is Planck's constant (6.626 * 10^-34 Js), and f is frequency.
3. To find the frequency, we use the speed of light (c) equation: c = λf, where λ is the wavelength. Rearrange the equation to find the frequency: f = c / λ.
4. Substitute the values and calculate the frequency: f = (3 * 10^8 m/s) / (6.71 * 10^-7 m) = 4.47 * 10^14 Hz.
5. Now, calculate the energy of one photon: E = (6.626 * 10^-34 Js) * (4.47 * 10^14 Hz) = 2.96 * 10^-19 J.
6. Finally, find the energy of 0.50 moles of photons: Energy = (0.50 moles) * (6.022 * 10^23 photons/mole) * (2.96 * 10^-19 J/photon) = 8.92 * 10^4 J.
So, the energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.92 * 10^4 Joules.
Learn more about photons: Which of the following could be the energy of a photon in the visible range? https://brainly.com/question/15946945
#SPJ11
The energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.93 x \(10^4\) J.
To find the energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm, we can use the following steps:
1. Convert the wavelength to meters: 671 nm = 671 x \(10^{(-9)}\) m
2. Calculate the energy of a single photon using Planck's equation: E = h * c / λ, where E is the energy, h is the Planck's constant (6.626 x \(10^{(-34)}\) Js), c is the speed of light (3.0 x \(10^8\) m/s), and λ is the wavelength in meters.
3. Calculate the total energy of 0.50 moles of photons by multiplying the energy of a single photon by Avogadro's number (6.022 x \(10^{(23)}\) particles/mole) and the number of moles (0.50).
Step-by-step calculation:
1. λ = 671 nm = 671 x \(10^{(-9)}\) m
2. E (single photon) = (6.626 x \(10^{(-34)}\) Js) * (3.0 x \(10^8\) m/s) / (671 x \(10^{(-9)}\) m) = 2.967 x \(10^{(-19)}\) J
3. Total energy = E (single photon) * 0.50 moles * (6.022 x \(10^{(23)}\) particles/mole) = (2.967 x \(10^{(-19)}\) J) * 0.50 * (6.022 x \(10^{(23)}\)) = 8.93 x \(10^4\) J
So, the energy of the light emitted by 0.50 moles of photons with a wavelength of 671 nm is approximately 8.93 x 10^4\(10^4\) J.
To learn more about moles, refer:-
https://brainly.com/question/26416088
#SPJ11
How much energy, in kj, is transferred between the system and surroundings when 250. 0 g of potassium fluoride is dissolved into water? the molecular mass of kf is 58. 10 g/mol. Give your answer as a positive number.
Answers
The energy, in kj, is transferred between the system and surroundings when 250. 0 g of potassium fluoride is dissolved into water, the molecular mass of kf is 58. 10 g/mol is 745 kJ per second thermodynamics
A thermodynamic device is the portion of the cosmos wherein observations are done, and the surroundings are the relaxation of the universe. The surroundings already has the entirety however the machine. We take into account that any tie desires to be broken energetically. The CO bond in acetone will therefore be broken with the aid of absorbing strength from the surrounding surroundings. presently, the acetone molecule's double bond between the CO atoms has a dissociation electricity of 745 kJ/mol. energy change will consequently be:Environmental strength is taken in at a fee of 745 kJ consistent with second (E = +745 kJ).The substance for which the chemical method KF stands is potassium fluoride. KF is the main supply of the fluoride ion for use in enterprise and chemistry, second best to hydrogen fluoride.A source of potassium is potassium fluoride, which is insoluble in water and used in oxygen-sensitive processes like metallic production.To learn more about thermodynamics, visit:
https://brainly.com/question/13669873
#SPJ4
Compounds are different than elements because compounds are–
Group of answer choices
a pure substance; elements are not.
not able to be broken down into parts.
made from the same type of atoms.
combinations of two or more elements.
Answers
Answer: Compound are different than elements because compounds are combinations of 2 or more elements.
Why do you think it is difficult to tell that a plate beneath it moving right now
Answers
Which of the following combinations will NOT result in a precipitate, according to
solubility rules?

Answers
The combination of ammonium chloride and sodium hydroxide will not give a precipitate. Option A
What is a precipitate?The solubility rules gives us the idea of the kind of combination of reactants that would lead to the formation of a precipitate. We can now apply that knowledge in this question.
These regulations are founded on the idea of solubility, or a substance's capacity to dissolve in a solvent, typically water.
These solubility rules provide a quick and easy way to predict the solubility of many ionic compounds in water.
Learn more about solubility rules:https://brainly.com/question/1297858
#SPJ1
Question: How can electrical conductivity be used to determine bond type? (In your CER make sure to also explain why different bonds behave differently)
1 claim
3 supporting evidence to the claim
3 reasonings supporting the evidence
Answers
Electrical conductivity helps in identify whether the bond is ionic or covalent bond.
Conductivity is the measure of the ease at which an electric charge or heat can pass through a material or also a solution. A conductor is a material through which electric current or charge can move freely through the material . Based on conductivity materials can be classified as metals, semiconductors, and insulators.
Along with that conductivity also helps us identify the type of bond that is present. If a compound cannot conduct electricity in its solid or liquid state, the it is considered to be a covalent bond. Because we can see that in covalent bond the conductivity is the least.
Whereas if a compound does conduct electricity when dissolved in solution or molten state, then it is an considered to be an ionic compound. Therefore we can say that ionic bonds are good conductors of heat and electricity.
To know more about Electrical conductivity
https://brainly.com/question/28916440
#SPJ1
A very good example of soft water is A. Distilled water B. Sea water C. Underground water D. Polluted water
Answers
Answer:
The answer is option A.
Distilled water
Since all the chemicals that will cause hardness in the water has been removed.
Hope this helps.
N the reaction methane + oxygen turns into carbon dioxide + water, the methane and oxygen are called
Answers
Answer:
Reactants
Explanation:
methane + oxygen turns into carbon dioxide + water
This is given as;
methane + oxygen --> carbon dioxide + water
In a reaction, all the substances before the arrow (the left side of the equation), are called Reactants!
please what is the formula for finding mole and give a worked example
Answers
Answer:
see explanation
Explanation:
number of moles = mass ÷ relative formula mass
e.g.
Calculate the number of moles of carbon dioxide molecules in 22 g of CO2.
Ar (relative atomic mass) of C = 12, Ar of O = 16
Mr (relative formula mass) of carbon dioxide = 12 + 16 + 16 = 44
number of moles = 22 ÷ 44 = 0.5 mol