Answers
Answer:
no way
Explanation:
more free points
Related Questions
which is the correct electron configuration for sodium?
Answers
Explanation:
Ne] 3s¹ is the answer your welcome
at which temperature does sf6 (boiling point 16°c at 1 atm) behave most ideally?
Answers
The optimal behavior of SF6 is achieved at a higher boiling point of 150C.
What factors determine the temperature?Temperature is a unit used to represent how hot or cold something is. It can be stated using the Celsius or Fahrenheit scales, among others. Temperature shows which way heat energy will naturally flow, i.e., from a hotter (body with a higher temperature) to a colder (body with a lower temperature) (one at a lower temperature).
What is the chemistry equivalent of writing temperature?Instead of using the written word to indicate temperature measurements, the ° symbol is typically used instead, with a space coming after the number but not before the temperature scale: To achieve 80 °C, the sample was heated.
To know more about Temperature visit:
https://brainly.com/question/15267055
#SPJ4
CHEMISTRY FORM THREE Exercise 2.1 1) Not all metals share the typical metal properties.name a metal that is: a. hard and strong b. malleable at room temperature

Answers
a) The strongest metal on the planet is tungsten.
When it comes to tensile strength, tungsten tops all other metals. One of the strongest metals known to man, tungsten has an ultimate strength of 1510 Megapascals.
In addition to having the greatest melting point of any unalloyed metal, tungsten boasts greater strength. Tungsten is frequently employed in electrical and military applications due to its strength.
Tin is a malleable, silvery-white metal that is highly flammable at ambient temperature.
Metal malleability is a complex subject. There is no objective test available to measure this feature, as we previously stated. We most frequently test it for hardness.
The most malleable and ductile metals are gold and silver. Pure gold and silver are too soft to be used to create items that will keep their shape.
Metals may be molded into different shapes, such as thin sheets or foils, without breaking or shattering because they are malleable. They are also ductile, making it simple to draw them into wires.
To learn more about metals from given link
https://brainly.com/question/4701542
#SPJ1
An aqueous solution of methanol (MM = 32.04 g/mol) has a molality of 8.83 m and a density of 1.15 g/mL. What is the molarity of methanol in the solution?
Answers
We have that the the molarity of methanol in the solution is mathematically given as
Morality=7.91mol/l
Chemical ReactionQuestion Parameters:
An aqueous solution of methanol (MM = 32.04 g/mol)
A molality of 8.83 m and a density of 1.15 g/mL.
Generally the equation for the is mathematically given as
\(Morality=\frac{moles of solute }{mass of the solvent}\)
where
Mass of solution=(100+282.2)
Mass of solution=1289.9
Volume=1289.9/1.15
Volume=1.115L
Therefore
\(Morality=\frac{moles of solute }{mass of the solvent}\)
Morality=8.83/1.115
Morality=7.91mol/l
For more information on Chemical Reactionvisit
https://brainly.com/question/11231920
HELP
Carla and Maurice are recording the temperatures of classrooms at their school, but they're using two different
thermometers. They plan to present their readings in both kelvins and degrees Celsius. Help the students convert their
temperatures from one scale to the other.
Type the correct answer in each box.

Answers
Answer:
1. 25.19
2. 301.57
Explanation:
it says it's correct...
The conversion of units of temperatures from one scale to the other which is measure by Carla and Maurice are given as.
298.34 K = 25.34°C
28.42°C = 301.42 K
How many units of temperature there?The temperature can be measure by using three units.
Kelvin abbreviate as KCelsius abbreviate °CFahrenheit abbreviate FRelationship between Kelvin (K) and Celsius (°C)
Let the value of temperature in Celsius (°C) unit as X °C, then value of this temperature in Kelvin (K) is,
X °C = 273 + X °C = (273 + X) K
Let the value of temperature in Kelvin (K) as Y K , then value of this temperature in Celsius (°C) is,
Y K = Y K - 273 = (Y - 273) °C
If 298.34 K
Then 298.34 K = 298.34 K - 273 = 25.34°C
If 28.42°C
Then, 28.42°C = 273 + 28.42°C = 301.42 K
To learn about temperature here.
https://brainly.com/question/13294753
#SPJ2
Are pressure and volume directly or inversely proportional
Answers
Pressure and volume can be regarded as the entity that is inversely proportional.
What is the relationship between Pressure and volume?It should be noted that this explanatin an be done using the law in chemistry which is the Boyle's law which states that, for a given amount of gas and constant temperature, the volume is inversely proportional to the pressure.
However the Equal quantities of all gases can be seen to have same number of molecules when subjected to the same temperature and pressure (Avogadro's law).
Learn more about volume at:
https://brainly.com/question/27710307
#SPJ1
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
Methanol is stored at the temperature of 60degree, the vapor pressure of methanol is 13.2kPa at 20degree and 347kPa at 100degree.
Answers
Answer: im only in 6th grad sory
Explanation:
Which refers to the passing of a wave through an object?
sound
O interference
O transmission
O frequency
O sound
Answers
The term that refers to the passing of a wave through an object is "transmission."
Transmission refers to the process by which a wave passes through an object or medium. In the context of sound, transmission occurs when sound waves travel through different substances, such as air, water, or solids.
When a sound wave encounters an object, it can be transmitted through it, reflected off it, or absorbed by it, depending on the properties of the object and the medium through which the sound is traveling.
For example, when you speak into a microphone, the sound waves produced by your voice travel through the air and are transmitted to the microphone's diaphragm. The diaphragm converts the sound waves into electrical signals, which can then be amplified and reproduced as sound through speakers.
In summary, transmission is the term used to describe the passage of a wave, such as a sound wave, through an object or medium. It is an essential concept in understanding how waves interact with their surroundings and how sound propagates through different materials.
for such more questions on transmission
https://brainly.com/question/18451537
#SPJ8
If you do not answer this you will feel bad for not answering it. Then hopefully you will come back and answer it.
N2SO4 + 2NaOH --> H2O + Na2SO4
How many molecules of water are produced if 2.0g of sodium sulfate are produced in the reaction above?
Question 3 options:
8.5 x 1021 molecules
8.5 x 1023 molecules
6.77 x 1022 molecules
2.0 x 1023 molecules
Answers
The balanced chemical equation for the reaction is:
N2SO4 + 2NaOH → Na2SO4 + 2H2O
From the equation, we can see that for every 1 molecule of sodium sulfate (Na2SO4), 2 molecules of water (H2O) are produced. Therefore, to find the number of molecules of water produced, we need to determine the number of molecules of sodium sulfate produced in the reaction.
To do this, we need to use the molar mass of sodium sulfate and Avogadro's number. The molar mass of sodium sulfate is 142.04 g/mol, and Avogadro's number is 6.022 x 10^23.
The number of molecules of sodium sulfate produced in the reaction can be calculated as follows:
(2.0 g) / (142.04 g/mol) = 0.01405 mol
And the number of molecules of water produced can be calculated as follows:
0.01405 mol x 2 molecules/mol = 0.0281 x 6.022 x 10^23 molecules/mol = 1.69 x 10^23 molecules
Round 1.69 to 2.0 and your answer is 2.0 x 1023 molecules
what is independent variable
Answers
Answer:
a variable (often denoted by x ) whose variation does not depend on that of another.
Explanation:
You can think of independent and dependent variables in terms of cause and effect: an independent variable is the variable you think is the cause, while a dependent variable is the effect. In an experiment, you manipulate the independent variable and measure the outcome in the dependent variable.For example, someone's age might be an independent variable. Other factors (such as what they eat, how much they go to school, how much television they watch) aren't going to change a person's age.
The point at where the water is changing phase
Answers
Answer:
the point at there the water is changing is titled "getting dressed"
What happens to the abundance of elements as their atomic numbers increase?
Answers
Answer:
Their abundance decreases.
Explanation:
Google :)
What are the 6 steps in the naturalization process?
Answers
What to do is On Form N-445, Notification of Naturalization Oath Ceremony, complete the questionnaire.Visit USCIS before your naturalisation ceremony and report there.Your Legal Resident Card must be returned (Green Card).To become an American citizen, you must take a Oath of Allegiance.
How long would it take to become a citizen in 2022?In a Nutshell:Application for Naturalization Form N-400 takes between 8 and 12 months to complete.On the USCIS website, keep records of your naturalisation application, and remain in touch wit your immigration attorney if you need any guidance.
What will it cost in 2022 to obtain American citizenship?$1,170.You have three options for paying the fee: cashier's check, personal check, or money order.You can pay with a credit card while submitting paperwork at the a USCIS lockbox by utilising Form G-1450, Permission for Card Transactions.
To know more about naturalization process visit:
https://brainly.com/question/12347547
#SPJ4
A vessel contains 2.00 mol of He, 4.50 mol of Kr, and 0.50 mol of N2 gases. If the partial pressure of He is 0.120 atm, what is the total pressure inside the vessel?
Answers
Considering the Dalton's partial pressure, the total pressure inside the vessel is 0.42 atm.
Dalton's partial pressureDalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
\(P_{T}\)= P₁ + P₂ + ... + Pₙ
where n is the amount of gases present in the mixture.
This relationship is due to the assumption that there are no attractive forces between the gases.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture. So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
\(P_{A}\)=\(x_{A}\)\(P_{T}\)
Total pressure inside the vesselIn this case, you know:
Amount of moles of He= 2 molesAmount of moles of Kr= 4.50 molesAmount of moles of N₂= 0.50 molesTotal amount of moles= Amount of moles of He + Amount of moles of Kr + Amount of moles of N₂= 2 moles + 4.50 moles + 0.50 moles= 7 molesPartial pressure of He= 0.120 atmThe partial pressure of gas He can be expressed as:
\(P_{He}\)=\(x_{He}\)\(P_{T}\)
Then, calculate the mole fraction of He as:
\(x_{He}\)= Amount of moles of He÷ Total amount of moles
\(x_{He}\)= 2 moles÷ 7 moles
\(x_{He}\)= 2/7
the total pressure can be calculated as:
0.120 atm= 2/7×\(P_{T}\)
0.120 atm÷ 2/7=\(P_{T}\)
0.42 atm= \(P_{T}\)
Finally, the total pressure is 0.42 atm.
Learn more about Dalton's partial pressure:
brainly.com/question/14239096
brainly.com/question/25181467
brainly.com/question/14119417
#SPJ1
Magnesium hydroxide reacts with chlorine to form magnesium chloride,
magnesium chlorate and water. How many grams of magnesium hydroxide is
needed to yield 8.00 moles of magnesium chlorate?
77.8 g Mg(OH)2
9178.1 g Mg(OH)2
2799.6 g Mg(OH)2
.823 g Mg(OH)2
How many grams of sodium sulfato pro
Answers
The grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g. None of the options provided match the calculated value of 466.64 g.
To determine the grams of magnesium hydroxide (Mg(OH)2) needed to yield 8.00 moles of magnesium chlorate (Mg(ClO3)2), we need to consider the balanced chemical equation for the reaction between magnesium hydroxide and chlorine.
The balanced equation is as follows:
2 Mg(OH)2 + 6 Cl2 → 2 Mg(ClO3)2 + 2 H2O
From the balanced equation, we can see that 2 moles of Mg(OH)2 react with 6 moles of Cl2 to produce 2 moles of Mg(ClO3)2.
Therefore, the stoichiometric ratio is 2 moles of Mg(OH)2 : 2 moles of Mg(ClO3)2.
To calculate the grams of Mg(OH)2 needed, we can use the stoichiometric ratio and the given moles of Mg(ClO3)2.
Given:
Moles of Mg(ClO3)2 = 8.00 moles
Using the stoichiometric ratio, we have:
8.00 moles Mg(ClO3)2 × (2 moles Mg(OH)2 / 2 moles Mg(ClO3)2) = 8.00 moles Mg(OH)2
To convert moles to grams, we need to multiply by the molar mass of Mg(OH)2.
The molar mass of Mg(OH)2 = (24.31 g/mol) + (2 * 16.00 g/mol) = 58.33 g/mol
Grams of Mg(OH)2 = 8.00 moles Mg(OH)2 × 58.33 g/mol = 466.64 g
Therefore, the grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g.
For more such questions on magnesium chlorate
https://brainly.com/question/12358640
#SPJ11
Question 23 of 25
Which of these is a disadvantage of using natural gas?
A. It is not considered safe to use in homes.
B. It burns less cleanly than other fossil fuels.
C. Scientists have developed new ways of extracting it.
D. Extraction through fracking can contaminate groundwater.
SUBMIT

Answers
Answer:
the answer is D. extraction through fracking can contaminate groundwater
The disadvantage of natural gas has been the contaminating groundwater due to fracking. Thus, option D is correct.
Natural gas has been the stored fossil energy that has been found buried deep inside the earth. The gas has consisted of a varying quantity of compounds with a maximum proportion of methane.
Natural gas has been nonrenewable, causing pollution with the burning. The release of greenhouse gases with the burning of natural gas results in affecting the environment.
Although there has been the presence of various extraction methods, natural gas results in contaminating groundwater due to fracking. Thus, option D is correct.
For more information about natural gas, refer to the link:
https://brainly.com/question/351648
Why do all elements have their own unique color? Please explain.
Answers
All the element have their own unique color because the every element have their own set of energy levels.
According to the bohr model of atoms : electrons exist at certain energy levels. when we give heat to electron , it gets excited and moves from lower energy level to higher energy level. the electron is less stable in higher energy level. when an electron returns from higher energy level to lower energy level it emits some energy in form of radiation. The wavelength of light depends upon energy level . and every elements have their own unique energy levels. The color for different element is different.
Thus, All the element have their own unique color because the every element have their own set of energy levels.
To learn more about element colors here
https://brainly.com/question/5809727
#SPJ1
If society took the threat of global warming seriously, what kinds of changes would you expect to see in how that society works?
Answers
Answer:
Global warming occurs a result of human activities which affects the environment negatively . It usually occurs over a long period of time. The global warming causes climate changes as a result of the ozone layer depletion.
If society took the threat of global warming seriously there will be changes in how humans handle the environment and the resources. Carbon emissions would be greatly reduced. Forest resources such as trees would also be treated specially and there would have been a reduction in mass deforestation as a result of urbanization
What is the mass of 2.25 moles of sulfuric acid (H2 SO4)?
Answers
Answer:
The molar mass of sulfuric acid (H2SO4) can be calculated by adding the atomic masses of its constituent atoms:
Molar mass of H2SO4 = 2(1.008 g/mol) + 1(32.06 g/mol) + 4(15.99 g/mol) = 98.08 g/mol
Therefore, the mass of 2.25 moles of H2SO4 is:
mass = number of moles x molar mass
mass = 2.25 moles x 98.08 g/mol = 220.68 g
So, the mass of 2.25 moles of sulfuric acid is 220.68 grams.
A monoprotic weak acid, HA , dissociates in water according to the reaction HA(aq)+H2O(l)↽−−⇀H3O+(aq)+A−(aq) The equilibrium concentrations of the reactants and products are [HA]=0.260 M , [H3O+]=4.00×10−4 M , and [A−]=4.00×10−4 M . Calculate the Ka value for the acid HA.
Answers
Answer:
Ka = 6.15x10⁻⁷
Explanation:
Ka is defined as dissociation constant in the equilibrium of a weak acid with water. The general reaction is:
HA(aq) + H₂O(l) ⇆ H₃O⁺(aq) + A⁻(aq)
And Ka is defined as the ratio between molar concentrations in equilibrium of products over reactants as follows:
Ka = [H₃O⁺] [A⁻] / [HA]
You don't take water in the equilibrium beacuse is a pure liquid
Replacing with the concentrations of the problem:
Ka = [H₃O⁺] [A⁻] / [HA]
Ka = [4.00x10⁻⁴] [4.00x10⁻⁴] / [0.260]
Ka = 6.15x10⁻⁷
How many protons, neutrons, and electrons does the following atom have?
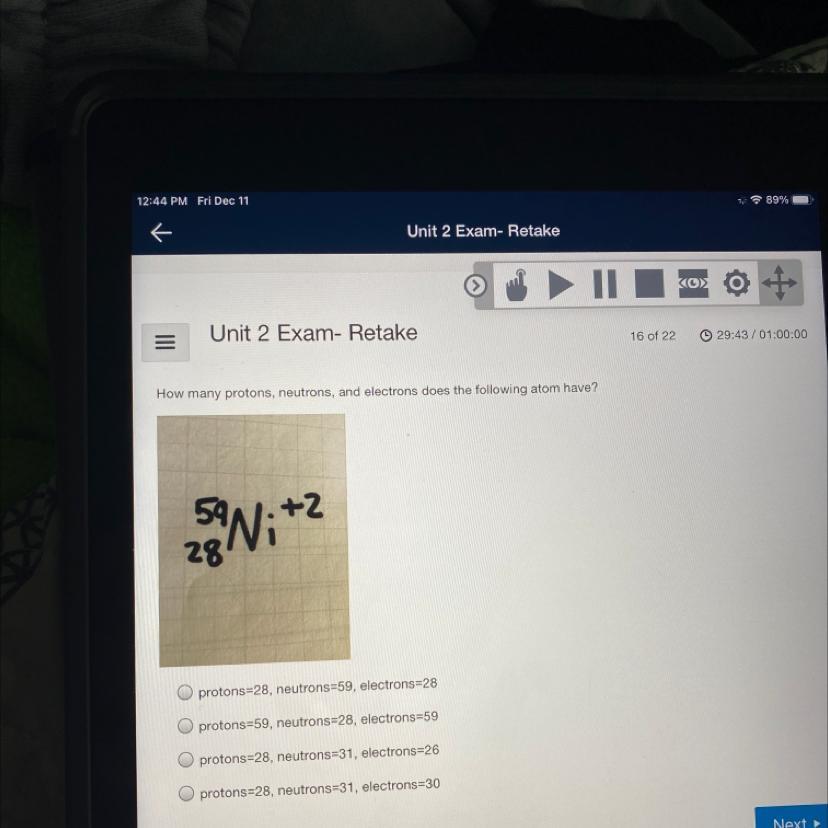
Answers
Answer:
Option C 28 Proton 31 neutron 26 elecrtons
Explanation:
Please read the question and choose the correct answer. Thank you.

Answers
The pH of the solution represent in the diagram, given that the solution contains 1 mole of H⁺ is 2 L is 0.3 (option B)
How do i determine the pH of the solution?We'll begin by obtaining the hydrogen ion, H⁺ concentration in the solution. This is shown below:
Mole of H⁺ = 1 moleVolume = 2 LHydrogen ion, H⁺ concentration = ?Concentration = mole / volume
Hydrogen ion, H⁺ concentration = 1 / 2
Hydrogen ion, H⁺ concentration = 0.5 M
Finally, we shall determine the pH of the solution. Details below:
Hydrogen ion concentration [H⁺] = 0.5 MpH of solution = ?pH = -Log [H⁺]
pH = -Log 0.5
pH = 0.3
Thus, we can conclude that the pH of the solution is 0.3 (option B)
Learn more about pH:
https://brainly.com/question/22983829
#SPJ1
A sample of a pure compound is analyzed and found to contain approximately 30 percent N and 70 percent O by mass. The formula for the compound could be
Answers
The formula of the compound, given that it contains 30 percent N and 70 percent O by mass is NO₂
How do i determine the formula of the compound?First, we shall list out the given parameters from the question. This is given
Percentage of nitrogen (N) = 30 percent Percentage of oxygen (O) = 70 percentFormula of compound =?The formula of the compound can be obtained as illustrated below:
Divide by their molar mass
N = 30 / 14 = 2.14
O = 70 / 16 = 4.375
Divide by the smallest
N = 2.14 / 2.14 = 1
O = 4.375 / 2.14 = 2
Thus, we can conclude from the above calculation that the formula of the compound is NO₂
Learn more about empirical formula:
https://brainly.com/question/9459553
#SPJ1
The formula of the compound that we have is NO2
What is the formula?We'll start by outlining the parameters that the question has provided. This is stated.
N = 30% in terms of nitrogen percentage.
oxygen percentage (O) = 70%
Compound's formula is =?
The compound's formula can be discovered as shown below:
Based on molar mass;
N = 30 / 14 = 2.14
O = 70 / 16 = 4.375
By the smallest number;
N = 2.14 / 2.14 = 1
O = 4.375 / 2.14 = 2
Thus the formula of the compound that we have to find is NO2.
Learn more about formula:https://brainly.com/question/14044066
#SPJ1
In a laboratory experiment a student found the pH of rain rain water sample to be 4.35 calculate H3O+ in the rainwater
Answers
The rainwater sample has a pH of 4.35, indicating that it is acidic. The concentration of \(H_3O^+\) ions in the sample is \(4.47 * 10^{-5} M\), which is higher than in neutral or basic solutions.
In a laboratory experiment, a student found the pH of a rainwater sample to be 4.35. In this context, the task is to calculate the concentration of hydronium ions (\(H_3O^+\)) in the given rainwater sample. The pH of the rainwater sample is an indication of how acidic it is. As per the pH scale, acidic solutions have a pH of less than 7. Since the pH of rainwater is acidic (pH = 4.35), it means that the concentration of \(H_3O^+\) ions is higher in the rainwater sample. To calculate the concentration of \(H_3O^+\) ions, we can use the following formula: pH = -log[\(H_3O^+\)], Where[\(H_3O^+\)] is the concentration of hydronium ions. By rearranging the above formula, we can get the concentration of \(H_3O^+\) ions as \([H_3O^+] = 10^{-pH}\). Substituting the given pH value of the rainwater sample in the above equation, we get\([H_3O^+] = 10^{-4.35} = 4.47 × 10^{-5} M.\)Therefore, the concentration of \(H_3O^+\) ions in the given rainwater sample is \(4.47 * 10^{-5} M.\)For more questions on acidic
https://brainly.com/question/4113018
#SPJ8
8. Sulfur has a first ionization energy of 1000 kJ/mol. Photons of what frequency are required to ionize one mole of Sulfur?
Answers
Answer:
the frequency of photons \(v = 1.509\times10^{39}Hz\)
Explanation:
Given: first ionization energy of 1000 kJ/mol.
No. of moles of sulfur = 1 mole
\(\Delta E_1 = 1000KJ/mol\)
We know that plank's constant
\(h = 6.626\times10^{-34} Js\)
Let the frequency of photons be ν
Also we know that ΔE = hν
this implies ν = ΔE/h
\(= \frac{10^6J}{6.626\times10^{-34} Js}\)
\(v = 1.509\times10^{39}Hz\)
Hence, the frequency of photons \(v = 1.509\times10^{39}Hz\)
calculate the molar internal energy of carbon dioxide at 298.15k , taking it's translational and rotational degrees of freedom into consideration
Answers
Answer:
Explanation:
To calculate the molar internal energy of a gas at a given temperature, you need to know the molar specific heat capacities at constant volume and constant pressure for the gas. These values are typically provided in tables of thermodynamic data, which can be found in various sources such as textbooks or online. Since you mentioned that you want to take the translational and rotational degrees of freedom into consideration, you will need to use the molar specific heat capacity at constant volume, which accounts for these degrees of freedom.
Once you have the molar specific heat capacity at constant volume for the gas, you can use the equation U = Cv * T, where U is the molar internal energy, Cv is the molar specific heat capacity at constant volume, and T is the temperature in kelvins. In your case, the temperature is 298.15 K, so plugging in the appropriate values and solving for U will give you the molar internal energy of carbon dioxide at that temperature.
It's important to note that the molar specific heat capacity at constant volume is typically a function of temperature, so you will need to use the appropriate value for the temperature you are interested in. Additionally, different sources may provide slightly different values for the molar specific heat capacity, so it's always a good idea to consult multiple sources to get a sense of the range of possible values.
How much heat is gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C? The specific heat of nickel is 0.443 J/g · °C.
Answers
Explanation:
To calculate the heat gained by nickel, we can use the formula:
q = m * c * ΔT
where q is the heat gained, m is the mass of the nickel, c is the specific heat of nickel, and ΔT is the change in temperature.
Given:
- Mass of nickel, m = 31.4 g
- Specific heat of nickel, c = 0.443 J/g · °C
- Change in temperature, ΔT = 64.2 °C - 27.2 °C = 37.0 °C
Substituting the values into the formula, we get:
q = (31.4 g) * (0.443 J/g · °C) * (37.0 °C)
Simplifying the calculation, we get:
q = 584 J
Therefore, the heat gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C is 584 J.
Who is the Scientist that came up with the idea of natural selection?
Answers
22.99 on the element sodium means the combined numbers of electrons and protons?
Answers
Answer:
The answer is 11 electron and 11 protons respectively
Explanation:
Since the sodium is in it neutral state, number of electron is the same as the number of proton, which is 11.
Answer:
Explanation:
you are welcome
