Answers
Related Questions
Calculate the amount of energy needed to raise the temperature of 10 g of gold from 30C to 40C.
Answers
The amount of energy needed to raise the temperature of 10 g of gold from 30°C to 40°C is 12.9 joules.
To calculate the amount of energy needed to raise the temperature of a substance, we can use the following formula;
Q = m × c × \(Δ_{T}\)
Where Q is the amount of energy (in joules), m is the mass of the substance (in grams), c is the specific heat capacity of the substance (in joules per gram per degree Celsius), and \(Δ_{T}\) is change in temperature (in degrees Celsius).
The specific heat capacity of gold is 0.129 joules per gram per degree Celsius.
Using this information, we can calculate the amount of energy needed to raise the temperature of 10 g of gold from 30°C to 40°C;
Q = 10 g × 0.129 J/g°C × (40°C - 30°C)
Q = 10 g × 0.129 J/g°C × 10°C
Q = 12.9 J
Therefore, the amount of energy needed is 12.9 joules.
To know more about amount of energy here
https://brainly.com/question/23775188
#SPJ1
PLZ HELP Question 14 of 25 What is the name for a representation of the physical world?
A. Model
B. Compound
C. Atom
D. Alloy SUBMIN
Answers
Answer:
Model
Explanation:
A model of anything is something you make to represent it in it's physical world form
A sample of helium gas occupies 12.4 L at 23°C and 0.956 atm. What volume will it occupy at 40°C and 0.956 atm? ___L
Answers
WHne the helium gas occupies 12.4 L at 23°C and 0.956 atm, then at 40°C and 0.956 atm the volume of the helium gas is 13.1 L.
How do you calculate the volume of helium gas ?We can use the combined gas law to solve this problem, which relates the pressure, volume, and temperature of a gas in a closed system. The well-known expression for the combined gas law is:
(P₁ x V₁) / T₁ = (P₂ x V₂) / T₂
We are given that P₁ = P₂ = 0.956 atm, V₁ = 12.4 L, T₁ = 23°C = 296 K, and T₂ = 40°C = 313 K. Putting these values into the gas formula, we obtain the following:
(0.956 atm x 12.4 L) / 296 K = (0.956 atm x V₂) / 313 K
Solving for V₂, we get:
V₂ = (0.956 atm x 12.4 L x 313 K) / (296 K x 0.956 atm) = 13.1 L
Therefore, the volume of the helium gas at 40°C and 0.956 atm is 13.1 L.
Learn more about combined gas law here:
https://brainly.com/question/30458409
#SPJ1
Each element consists of atoms unique to that element. What has each element been assigned that uniquely identifies it
Answers
because im in a filed of dandelions wishing on everyone that youll be mine
If the specific heat of a material is 0.416 J/(g°C) and sample absorbs 50 J when it is heated from 30°C to 50°C what is the mass of the sample?
Answers
The mass of the sample of the material is 0.1664 g.
What is specific heat?The amount of energy needed to raise the temperature of one gram of a substance by one degree Celsius.
By the formula of specific heat
\(Q = mc \Delta T\)
Given, that the specific heat (c) of material is 0.416 J/gC
The difference in temperatures is 30°C to 50°C
The mass=?
Q = heat, 50 J
Putting the values in the equation
\(\rm 50\;J = m \times 0.416 J/g^\circ C \times (50 - 30)\\\\m = \dfrac{0.416 J/g^\circ C \times 20}{50} = 0.1664 g\)
Thus, the mass of the samples is 0.166 g.
Learn more about specific heat
https://brainly.com/question/11297584
#SPJ1
how many grams of potassium chloride can be produced from 50.0 g chlorine and 50.0 g potassium bromide? which one is the limiting reactant and which one is the excess reactant?
Answers
We can conclude as chlorine is the excess reactant and potassium bromide is the solute. Just 31.3 g of KCl, the mass produced from solute, can be made by 50.0 g of chlorine & 50.0 grams of potassium bromide.
What is a reactant?The substance which is present when a chemical reaction first begins is known as a reactant. Products refer to the material or substances to a right of the arrow. A substance that's also present following a chemical reaction is known as a product.
Provide a product and reactant example.In the first, sodium (Na) and chlorine (Cl) are the reactants, and the finished product being sodium chloride, called table salt (NaCl N a C l ).
To know more about reactant visit:
https://brainly.com/question/17096236
#SPJ9
Question 1 (True/False Worth 2 points)
(02.02 MC)
Salt dissolving in water is a physical change.
O True
O False
Answers
Answer:
True
Explanation:
Any substance dissolving a solid (salt) in liquid (water) is a physical change.
Salt in water, is a physical change because only the state of the matter has changed.
Answer:
true
Explanation:
uwu
True or false? Ionic compounds form regular crystals because their ions arrange themselves in a stable lattice structure.
Answers
Answer:
TRUE!!!!!!!!!!
Explanation:
Hope this helps!!! :)
How many Liters of space will a 70.0g sample of CO2 occupy? You must show all work to receive full credit.
Answers
Answer: the answer is 35.6
Explanation:
Look up the answer but take out you must show all your work and resources should pop up I’m on the same question now for chemistry so I’m putting 35.6L
This is for chemistry
I need help for this question

Answers
Answer:
what is the quetion.......
Explanation:
what does Le châteliers principle state?
Answers
Hope this helps!
How do ionic bonds form between atoms?
Answers
In a polar covalent bond, electrons are shared ___________.
A. equally
B. unequally
C. between non-metals with similar electronegativities
D. between a metal and a non-metal
Answers
In a polar covalent bond, electrons are shared unequally. The correct alternative is option b.
A polar covalent bond is a type of bond in which electrons are shared unequally between two atoms. This unequal sharing of electrons results in a partial negative charge on one side of the bond and a partial positive charge on the other side.
This charge separation creates a dipole, which is a molecule with two poles.
The unequal sharing of electrons is due to the difference in electronegativity between the two atoms. The atom with the higher electronegativity will have a greater attraction for the electrons and will therefore have a partial negative charge, while the atom with the lower electronegativity will have a partial positive charge.
To know more about polar covalent bond here:
https://brainly.com/question/28955235#
#SPJ11
A person has a mass of 70.0 kg on Earth,
what would their mass be on the Moon,
which has 1/6 the gravity of Earth?
Answers
Answer:
11.67 lbs
Explanation:
hi. can you help me

Answers
2. Helium, He, 4, 2 and 2
3. Beryllium, 4, 5 and 5
4. Nitrogen, 7, 14 and 7
5. Neon, 10, 20 and 10
6. Aluminum, 13, 27 and 13
7. Sulfur, S, 16, 16 and 16
8. Boron, B, 5, 6 and 5
9. Oxygen, O, 8, 16 and 8
10. Sodium, 11, 12 and 11
11. Silicon, Si, 14, 14 and 14
12. Chlorine, 17, 35 and 17
13. Lithium, Li, 3, 7 and 3
14. Carbon, C, 6, 6 and 6
15. Fluorine, 9, 9 and 10
16. Magnesium, 24, 12 and 12
17. Phosphorus, 15, 31 and 15
18. Argon, Ar, 40, 18 and 18
19. Calcium, 20, 40, 20, 20 and 20
20. Potassium, K, 19, 39, 19, 20 and 19
Hope it helps, the element names are to help you with locating it, the numbers are to fill in.
15. A pipe leaks water at a rate of 1.24 mL/s. What is the rate of the water leak in L/hr?
ALL
Answers
Answer:
4.46
Explanation:
1.24 ml= 0.00124 L
3600 s=1 hr
0.00124 x 3600=4.46
Matter are anything that is made up of atoms. The quantity of matter can be observed only on the basis of mass and volume calculation. Therefore, the rate of the water leak in L/hr is 4.46L/hr.
What is matter?Matter is a substance that has some mass and can occupy some volume. The matter is mainly used in science. Matter can be solid, liquid or gas.
Matter is anything that is made up of atoms. Anything around us that can be physically seen and touched are matter. Ice, water and water vapors are example of matter.
So as we saw that matter has some mass so mass can be measured in gram only. Mass can also be represented as number of molecules. We also saw that matter occupy some volume and that volume is measured only in liter.
1.24 ml= 0.00124 L
3600 s=1 hr
0.00124 x 3600=4.46L/hr
Therefore, the rate of the water leak in L/hr is 4.46L/hr.
To learn more about matter, here:
https://brainly.com/question/4562319
#SPJ2
Calculate the mass of oxygen gas required tooccupy a volume of 6 L at a pressure of 20.9kPa and a temperature of 37◦C.1. 0.779 g2. 1.56 g3. 0.408 g4. 13.1 g5. 0.0487 g
Answers
The mass of oxygen gas required to occupy a volume of 6 L at a pressure of 20.9 kPa and a temperature of 37°C is 0.155 g, which is closest to option 2 (1.56 g). Option 2 is correct.
To solve this problem, we can use the ideal gas law, which relates the pressure, temperature, volume and the number of moles of a gas:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, as well as T is the temperature. We rearrange this equation to solve for the number of moles of gas:
n = PV/RT
We know the pressure (20.9 kPa), volume (6 L), temperature (37°C = 310 K), and we are looking for the number of moles of oxygen gas. The gas constant R is 8.31 J/(mol·K). Molar mass of oxygen gas (O₂) will be 32 g/mol.
n = PV/RT = (20.9 kPa)(6 L)/(8.31 J/(mol·K) × 310 K)
= 0.00487 mol
To calculate the mass of oxygen gas, we can use the molar mass:
mass = n × molar mass
= 0.00487 mol × 32 g/mol
= 0.155 g
Hence, 2. 1.56 g is the correct option.
To know more about ideal gas law here
https://brainly.com/question/13821925
#SPJ4
A chemist collected 56.1 ml of gas in an open manometer. the next day, the chemist noted that the volume had changed to 57.9 ml and the barometer reading was 99.4 kpa. the temperature had not changed. what had been the barometer reading on the previous day when the gas was collected?
Answers
The previous day's barometer reading when the gas was collected would have been 100.7 kPa. The difference in volume (57.9 ml - 56.1 ml = 1.8 ml) indicates a decrease in gas volume.
Since the temperature remained constant, according to Boyle's Law, the pressure and volume of a gas are inversely proportional. Therefore, the decrease in volume caused an increase in pressure. By subtracting the change in pressure (1.3 kPa) from the current barometer reading (99.4 kPa), we find the previous day's barometer reading to be 100.7 kPa. Given that the volume increased from 56.1 ml to 57.9 ml while the temperature remained constant, the pressure decreased. According to Boyle's Law, the pressure and volume of a gas are inversely proportional. By calculating the difference in volume (57.9 ml - 56.1 ml = 1.8 ml) and assuming an ideal gas, we can determine that the previous day's barometer reading was 100.7 kPa (99.4 kPa + 1.3 kPa).
learn more about gas here:
https://brainly.com/question/14812509
#SPJ11
O2(g)+4H+(aq)+2Zn(s)→2H2O(l)+2Zn2+(aq) Indicate the half-reaction occurring at Cathode.
Answers
When the chemicals in the reaction are in aqueous solution, the half-reaction approach performs better than the oxidation-number method. Because the aqueous solution is usually acidic or basic, hydrogen ions or hydroxide ions are present. In general, the half-reactions are first balanced independently by atoms.
The overall cell reaction given in the question is
\(O{2}\)(g)+ 4H+(aq)+ 2Zn(s) → 2\(H_{2} O\)(l)+ 2\(Zn_{2}\)+(aq)
So Zn (s) is oxidized to \(Zn^{2+}\) at anode. Since anode is the half cell where oxidation happens.
At anode: 2 Zn (s) → 2\(Zn^{2+}\)+(aq)+ 4e-
At cathode reduction will happen. Here, \(O_{2}\) (g) is reduced by 4e- electrons and combine with 4H+ to form 2\(H_{2}O\).
Thus, cathode half cell reaction can be represented as
At cathode: O2 (g) + 4H+ (aq)+ 4e- → 2\(H_{2}O\)
Thus, overall reaction is
\(O_{2}\)(g)+ 4H+(aq)+ 2Zn(s) → 2\(H_{2}O\)(l)+ \(Zn^{2+}\)(aq)
Learn more about half reaction here:
https://brainly.com/question/32677817
#SPJ12
The half-reaction occurring at Cathode indicate Zn (s) is oxidized to Zn2+ at anode. Since anode is the halfcell where oxidation happens.
The overall cell reaction given in the question is
O2(g)+ 4H+(aq)+ 2Zn(s) → 2H2O(l)+ 2Zn2+(aq)
An oxidation or reduction reaction is a half-cell process. We lose electrons in one and acquire electrons in the other. These processes occur in an electrochemical cell in which we lose electrons at the anode where oxidation occurs and gain electrons at the cathode where reduction occurs.
So Zn (s) is oxidized to Zn2+ at anode. Since anode is the halfcell where oxidation happens.
At anode: 2 Zn (s) → 2Zn2+(aq)+ 4e-
At cathode reduction will happen. Here O2 (g) is reduced by 4e- electrons and combine with 4H+ to form 2H2O.
Thus cathode half-cell reaction can be represented as
At cathode: O2 (g) + 4H+ (aq)+ 4e- → 2H2O
Thus, overall reaction is O2(g)+ 4H+(aq)+ 2Zn(s) → 2H2O(l)+ 2Zn2+(aq)
Learn more about Half-cell here,
https://brainly.com/question/28303395
#SPJ12
Which formula compares a sample of a gas under two different conditions of pressure and volume, if the temperature is constant?
⚪︎ x/y = k
⚪︎ xy = k
⚪︎ PV = k
⚪︎ V/T = k
⚪︎ P1V1 = P2V2

Answers
Answer:
Explanation:
V/t
A 25. 00 ml sample of acetic acid containing phenolphthalein indicator is titrated with 0. 1067 m naoh. The solution changes color after 30. 07 ml naoh has been added. What is the concentration of the acetic acid before titration?.
Answers
The concentration of the acetic acid before titration is 0.128M
For weak acid and strong base titration-
Moles of acetic acid = moles of NaOH
Given concentration of NaOH = 0.1067M
Volume of NaOH = 30.07ml = 0.03007L
Using the formula, Concentration = Moles/Volume
Moles of NaOH = concentration X volume = 0.1067 X 0.03007 = 0.0032
Therefore, moles of acetic acid = 0.0032mole
Using the formula, Concentration = Moles/Volume
Concentration of acetic acid = 0.0032/0.025 = 0.128M
Learn more about titration -
https://brainly.com/question/23307058
#SPJ4
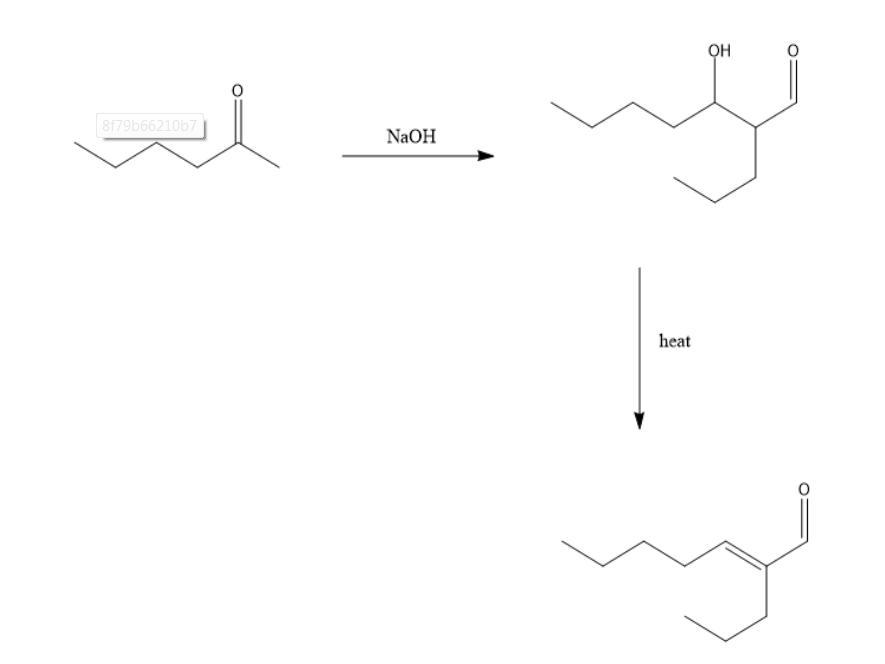
Draw the structures of the aldol addition and condensation products of pentanal
Answers
The structures of the aldol addition and condensation products of pentanal are attached as an Image with this answer.
What is Aldol Addition ?
An aldol addition is a reaction that occurs between the enolate of an aldehyde or ketone and the alpha-carbon of another aldehyde or ketone to form a beta-hydroxy aldehyde or ketone.
The term aldol condensation has to do with a reaction in which a nucleophile attacks the carbonyl group of an aldehyde or a ketone to convert it to the enolate from which attacks another aldehyde or ketone at the carbonyl carbon to form the required product.
The product obtained in the process is shown in the image attached with the answer.
To know more about Aldol Addition
https://brainly.com/question/27181492
#SPJ4
Tres diferencias entre los líquidos y los sólidos
Answers
Answer: Density, Volume, Shape
Use your knowledge of atomic calculations to complete the chart.
Element
Protons
Neutrons
Electrons
Atomic
Number
3
15
Atomic
Mass
7
31
35
19
28
31
P
ci
Ni
K
Ag
H
39
19
-18
47
OK
1
1
14
14
06
3
Si
w
74
1010
Ne
10
10
Answers
Answer:
For hydrogen. H
Explanation:
Atomic number is 1
Proton number is 1
Mass number is 2
Neutron number is 1
Give reasons :
Concentrated H2SO4 cannot be used instead of dilute H2SO4 in laboratory preparation of hydrogen gas why ?
Answers
Which of the following statements about buffers is false? a) a buffer resists changes in pH upon addition of small amounts of strong acids and bases b) a buffer solution can react with either HjOor OH ions c) a buffer solution resists change in pH upon small dilutions d) a buffer always consists of a weak acid and a soluble ionic salt of the weak acid e) most body fluids contain natural buffer systems
Answers
The false statement about buffers is: d) a buffer always consists of a weak acid and a soluble ionic salt of the weak acid.
Buffers are solutions that can resist changes in pH when small amounts of acids or bases are added. They typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). The weak acid and its conjugate base are present in equilibrium, allowing the buffer to accept or donate protons to maintain a relatively constant pH. Option d is false because a buffer can consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. It is not necessary for a buffer to always include a soluble ionic salt.
To learn more about buffers click here: brainly.com/question/31847096 #SPJ11
how many joules of heat are absorbed when 1000g of water is heated from 18Celsius to 85celsius?
Answers
Answer + Explanations
Calculate heat absorption using the formula:
Q = mc∆T
Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and ∆T is the change in temperature.
The heat absorbed is calculated by using the specific heat of water and the equation ΔH=cp×m×ΔT. 4. Water is vaporized to steam at 100oC. The heat absorbed is calculated by multiplying the moles of water by the molar heat of vaporization.
You can do this easily: just multiply the heat capacity of the substance you're heating by the mass of the substance and the change in temperature to find the heat absorbed.
To calculate the amount of heat released in a chemical reaction, use the equation Q = mc ΔT, where Q is the heat energy transferred (in joules), m is the mass of the liquid being heated (in kilograms), c is the specific heat capacity of the liquid (joule per kilogram degrees Celsius), and ΔT is the change in ...
Q = mc∆T. Q = heat energy (Joules, J) m = mass of a substance (kg) c = specific heat (units J/kg∙K) ∆ is a symbol meaning "the change in"
Precisely, water has to absorb 4,184 Joules of heat (1 calorie) for the temperature of one kilogram of water to increase 1°C. For comparison sake, it only takes 385 Joules of heat to raise 1 kilogram of copper 1°C.
A reaction that absorbs heat is endothermic. Its enthalpy will be positive, and it will cool down its surroundings. This reaction is exothermic (negative enthalpy, release of heat).
Quantitative experiments show that 4.18 Joules of heat energy are required to raise the temperature of 1g of water by 1°C. Thus, a liter (1000g) of water that increased from 24 to 25°C has absorbed 4.18 J/g°C x 1000g x 1°C or 4180 Joules of energy.
Hydrogen gas caught fire quickly in the Hindenberg accident. In comparison, neon gas and helium gas are nonreactive. This is why helium is safe for aircraft and neon is safe for electrical signs. Which statement best explains why helium and neon have similar chemical properties?
Answers
Answer:
Helium and Neon have similar chemical properties because both of them completely fill the outer shell of their atoms' electrons, so there is nothing to share with other atoms, neither with the same element nor with any other element.
Explanation:
Helium and Neon are both noble gases . As elements react, their atoms, by losing, acquiring, or sharing electrons, complete their outer shells. Noble gas atoms already have full outer shells, so there is no tendency for them to lose, gain, or share electrons. There are incomplete outer shells of atoms of group 1 and 7 elements (so they are reactive)
Chemical properties of HELIUM and NEON -:
In group 8(or 0) of the periodic table, on the far right side, the Noble Gases are contained. Helium, neon, argon, krypton, xenon and radon are their names. They are extremely colourless and unreactive. They do not form bonds, so they still remain as single (monatomic) atoms. There is very little chemical reactivity in them.Hence, helium and neon have similar chemical properties as they are the most stable due to having the maximum number of valence electrons their outer shell can hold.
Answer this please need help right now
Objects
1. Plastic Bottle
2. Plastic Bottle

Answers
Potential energy : energy obtained due to height or position (the force of gravity)
PE = mhg
g = acceleration due to gravity (m/s²)
h = height (m)
m = mass (kg)
1. Known
m = 0.5 kg
h = 1 m
g = 9.8 m/s²
then :
\(\tt PE=0.5\times 1\times 9.8=4.9~J\)
Kinetic energy is the energy gained by an object's motion
KE = 1/2 mv²
2. To find KE, the required data are the mass and velocity
in this table, you do not display the velocity
2. Calculate the mass of Kool-Aid needed to make 0.1 L solutions at the following concentrations: a. 0.1 M b. 0.2 M c. 0.3 M d. 0.4 M e. 0.5 M
