Answers
Answer:
It equals 826
Explanation:
Cos that's ur moms weight in pounds
Related Questions
__ SnCl4 → __ Sn + __ Cl2
A. 221
B. 132
C. 121
D. 112
Answers
Answer:
Sn (s) + 2 Cl2 (g) → SnCl4 (l)
This is an oxidation-reduction (redox) reaction:
4 Cl0 + 4 e- → 4 Cl-I
(reduction)
Sn0 - 4 e- → SnIV
(oxidation)
Cl2 is an oxidizing agent, Sn is a reducing agent.
Reactants:
Sn
Names: Tin source: wikidata, accessed: 2019-09-07source: ICSC, accessed: 2019-09-04source: NIOSH NPG, accessed: 2019-09-02, Sn source: wikidata, accessed: 2019-09-07, Element 50 source: wikidata, accessed: 2019-09-07
Appearance: White crystalline powder source: ICSC, accessed: 2019-09-04; Gray to almost silver-white, ductile, malleable, lustrous solid. source: NIOSH NPG, accessed: 2019-09-02
Cl2
Names: Chlorine source: ICSC, accessed: 2019-09-04source: NIOSH NPG, accessed: 2019-09-02, Molecular chlorine source: NIOSH NPG, accessed: 2019-09-02
Appearance: Greenish-yellow compressed liquefied gas with pungent odour source: ICSC, accessed: 2019-09-04; Greenish-yellow gas with a pungent, irritating odor. [Note: Shipped as a liquefied compressed gas.] source: NIOSH NPG, accessed: 2019-09-02
Products:
SnCl4 – Tetrachlorostannane source: wikipedia, accessed: 2019-09-28source: wikidata, accessed: 2019-09-02, Tin tetrachloride source: wikipedia, accessed: 2019-09-28source: wikidata, accessed: 2019-09-02source: ICSC, accessed: 2019-09-04, Tin(IV) chloride source: wikipedia, accessed: 2019-09-28
Other names: Stannic chloride source: wikipedia, accessed: 2019-09-28source: wikidata, accessed: 2019-09-02source: ICSC, accessed: 2019-09-04, Tin(iv) chloride (anhydrous) source: ICSC, accessed: 2019-09-04
Appearance: Colorless to slightly yellow fuming liquid source: wikipedia, accessed: 2019-09-28; Colourless or slightly yellow fuming liquid with pungent odour source: ICSC, accessed: 2019-09-04
If 35.0 grams of coal (carbon) burns in 58.5 grams of oxygen gas, how many grams of carbon dioxide can be produced? Describe the steps.
C + O2 → CO2
Answers
Answer:
0.59
Explanation:
Is 0.59 because you are dividing 35.0g by 58.5 which will than give you 0.59
Based on the strength of their intermolecular forces, you would expect CH3-O-CH3 to have ___ boiling point compared to CH3CH2OH.
A. an equal
B. a lower
C. a higher
Answers
Answer:
higher
Explanation:
as CH3CH2OH has an O-H bond, it has significantly more IMF caused by the hydrogen bond between CH3CH2OH molecules. This means its harder to pull apart CH3CH2OH molecules as they are very attracted to one another, thereby increasing the boiling point.
Balance the equation: "Sodium oxide reacts with water to form sodium hydroxide and hydrogen." I can't find the correct coefficients to balance the equation. I have written my unbalanced equation in the file attached.

Answers
Answer:
So, sodium oxide + water = sodium hydroxide + hydrogen is written Na2O+H2O-->NaOH+H.
To balance the equation it should read NaO+H2O-->2NaOH
Explanation:
Na2O+H2O-->2NaOH+0H2.......the 0H2 is dropped because there is no value
The correct balance of equation will be
Na2O + H2O - - > 2NaOH
What is a balanced chemical equation?A chemical equation where the number of atoms on both the reactant side and the product side of the reaction are equal is called a balanced chemical equation.
In a balanced chemical equation, not only the number of atoms but the charge and the mass is the same on both sides of the equation.
A chemical equation needs to be balanced to validate the law of conservation of mass. The law of conservation of mass states that the 'mass can neither be created nor destroyed in a chemical reaction'.
If the chemical equation is not balanced, it will go against the fundamental law of law of conservation of mass.
Thus, the chemical equations need to be balanced.
Therefore to balanced the given chemical equation, we equate the number of atoms on both the reactant and the product side of the reaction.
It will be written as
Na2O + H2O - - > 2NaOH
Read more about balanced chemical equations, here
https://brainly.com/question/8062886
#SPJ5
we make a solution of cu(ch3co2)2(aq) with a concentration of 0.0880 m and add a 7.0 g chunk of silver metal. what is the equilibrium concentration of silver ions?
Answers
The equilibrium concentration of silver ions in this solution cannot be determined without knowing the rate constants of the reaction between silver and Cu(CH3CO2)2.
What is equilibrium concentration?The level of concentration of an identifiable chemical species in an environment at equilibrium is known as equilibrium concentration. A different name for it is the constant state percentage. The equilibrium constant is the measurement of the response and the initial amounts of both reactants and byproducts in the system define this level of concentration.
The full name of Cu(CH3CO2)2 compound is dimethyl carbonate cupric complex in aqueous solution.
The response is:
Cu(s) + 2AgCH3CO2(aq) = Cu(s) + Cu(CH3CO2)2(aq) + Ag(s)
Cu(CH3CO2)2 has a molecular weight of 0.0880 moles.
The silver content is 7.0 g/108 g/mol, or 0.0648 moles of silver.
Cu(CH3CO2)2 and Ag have a mole ratio of 1:2, meaning that 0.0880 moles of Cu(CH3CO2)2 will react with 0.1760 moles of Ag.
Because there are fewer moles of Ag than there are of Cu(CH3CO2)2, all of the Cu(CH3CO2)2 will react with whereas some of the Ag will not.
The total amount of Ag that won't undergo any reactions is equal to 0.1112 moles of Ag (0.1760 moles - 0.0648 moles).
The unprocessed Ag weighs 11.9 g, or 0.1112 moles x 108 g/mol.
Learn more about equilibrium concentration, here:
https://brainly.com/question/16645766
#SPJ4
Pure gold has a density of 19.3 g/cm^3 . How large would a piece of gold be if it
had a mass of 318.97 g?
Answers
Density = Mass/Volume but it can also be rearranged to:
Volume = Mass/Density
Given in the question:
Mass - 318.97 g
Density - 19.3 g/cm3
Calculation
Density = Mass/Volume
= 318.97/19.3
= 16.52 \(cm^{3}\)
Therefore, the volume of the gold is 16.52 \(cm^{3}\)
Question 11
1 pts
If a calcium atom has 20 protons, 21 neutrons, and 20 electrons, what is its atomic
number?
40
0 21
O 20
41
Answers
Andrew and Sullivan were arguing over bond lengths. Andrew said that F-CI has a shorter bond
length than F-H because F and Cl have greater electronegativity and therefore form a shorter
bond. Sullivan disagrees, stating that electronegativity has nothing to do with bond length.
Who's right? Why?
Answers
The ability of an atom to draw in the shared pair of electrons is measured by its electronegativity. For calculating electronegativity, people frequently utilize the Pauling scale.
What is electronegativity?When an electron is added to a neutral atom in a gaseous state to create a negative ion, the energy of the atom changes (in units of kJ/mole). In essence, it conveys the atom's propensity to pick up an electron.
In the modern periodic table's 17th group, fluorine is located above chlorine. Despite having seven electrons in its valence shell, it is smaller than an atom of chlorine.
The electron density rises as a result. Fluorine has a higher electronegativity because of its high electron density, which can draw in a shared pair of electrons.
Therefore, The ability of an atom to draw in the shared pair of electrons is measured by its electronegativity. For calculating electronegativity, people frequently utilize the Pauling scale.
To learn more about electronegativity, refer to the link:
https://brainly.com/question/10531792
#SPJ1
Why is Newton's first law of motion is sometimes called the law of inertia
Answers
Answer:
The reason why Newtons first law of motion is sometimes called the law of inertia is because it states that if the object is in motion, it will not rest unless an unbalanced force acts on the object.
please answer the question

Answers
Answer:
Stamens
Explanation:
What is the function of the structure labeled Y?
to keep oxygen-rich blood and oxygen-poor blood flowing
to filter waste materials from oxygen-poor blood
to filter waste materials from oxygen-rich blood
to keep oxygen-rich blood and oxygen-poor blood separate
Answers
The function of Y would be to keep oxygen-rich blood and oxygen-poor blood separate. The last option is the correct one.
Function of the SeptumY is the septum
The septum is a structure of the heart that separates the left atrium from the right atrium as well as the two ventricles.
Thus, the structure prevents oxygenated blood from mixing with deoxygenated blood.
More on the heart's septa can be found here: https://brainly.com/question/17295714
#SPJ1

Why krypton and noble gas are not used in airships using the periodic table?
Answers
Answer:
Since the noble gases are unreactive or inert, they are safe to use. Helium is used to fill balloons and airships, because it is much lighter that air and it will not catch fire. Neon is used in advertising signs. It will give red glow, but the color can be changes by mixing it with other gases.
Explanation:
LOTS OF POINTS FOR WHOEVER ANSWERS THIS
Place the elements Lithium (Li), Beryllium (Be), and Potassium (K) in order from lowest electronegativity energy to the highest. Explain with details and use the Coulomb's Law to back up your statement
Answers
You should probably rephrase this:
According to Coulomb's Law, as the number of protons in an atom increases (or atomic number), the nuclear energy of atoms will increase, pulling electrons closer.
I think the order would be Be, Li, K but feel free to check online before you submit any work with this.
What are the 4 common elements that form covalent bonds?
Answers
Answer:
The four (4) most important elements found in cells that form covalent bonds are carbon, hydrogen, oxygen and nitrogen.
Explanation:
Why does lead affect the brain and nervous system?
Answers
Answer:
Lead may disrupt the functioning of mitochondria in the developing brain. Because the mitochondria are important for energy production within a neuron, a change in their function may damage the cell. Lead may also affect brain function by interfering with neurotransmitter release and synapse formation.
Explain why only one type of seismic wave was recorded at location B. (1 sentence only!)
Answers
Answer:
p wave
Explanation:
The P wave is designated the primary preliminary wave because it is the first to arrive at a seismic station after an earthquake. It travels at a speed usually less than 6 kilometers per second in the Earth's crust and jumps to 13 kilometers per second through the core.
I WILL GIVE U BRAINLIEST

Answers
Answer: YEAH ITS the third one
Explanation: duhh
pls answer question will mark brainliset tyty question is in the picvture

Answers
Answer:
d
Explanation:
exotic species, took this last week.
C. Use the data provided in Table 2 to complete the following.
Sketch a phase diagram for O₂. The diagram should be roughly to
scale and include the Triple point and Critical point.

Answers
The triple point can be seen from the graph that has been attached here.
What is the triple point?The triple point is a special combination of temperature and pressure where the equilibrium of a substance's three phases—solid, liquid, and gas—occurs. The transition between phases happens with no discernible net change in the substance because the solid, liquid, and gas phases are in perfect equilibrium at the triple point.
A crucial reference point in thermodynamics, the triple point is frequently used to specify temperature scales and calibrate thermometers. Each substance has a specified value for temperature and pressure.
Learn more about triple point:https://brainly.com/question/29017350
#SPJ1

hi. i need help with this. i lack with this subject

Answers
Answer: It's the circulatory system
Explanation:
Scott and his classmates were looking at an animal cell under a microscope. They are trying to locate the cell's DNA, which is found in its chromosomes. Where should Scott look to find the chromosomes?
In the mitochondria
In the cell nucleus
In the cytoplasm
In the cell vacuole
Answers
What would you predict, the solubility of KHT (solid) in pure water compared with the solubility of KHT (solid) in a 0.1 M KCl solution, which one will be higher? Explain your answer.
Answers
The solubility of KHT (solid) in pure water compared with the solubility of KHT (solid) in a 0.1 M KCl solution is predicted to be higher in the 0.1 M KCl solution. This is because the KCl solution has a higher ionic strength, increasing the solubility of ionic compounds like KHT.
Let's understand this in detail:
What is solubility?
Solubility is defined as the ability of a substance to dissolve in a particular solvent under certain conditions. It measures the maximum amount of solute that can be dissolved in a given amount of solvent at a particular temperature, pressure, and other conditions.
Solubility of KHT in pure water:
KHT (Potassium hydrogen tartrate) is a weak acid salt that has low solubility in pure water. The solubility of KHT in pure water is affected by various factors such as temperature, pH, and pressure. The solubility of KHT in pure water is around 4.4 g/L at room temperature.
Solubility of KHT in 0.1 M KCl solution: The solubility of KHT in a 0.1 M KCl solution is predicted to be higher than in pure water. KCl is an ionic salt dissociating in water to produce K+ and Cl- ions. The presence of KCl increases the ionic strength of the solution. This ionic strength improves the solubility of other ionic compounds, such as KHT. KHT has a higher solubility in a 0.1 M KCl solution than in pure water due to this reason.
#SPJ11
Learn more about solubility: Explain how you would find the solubility of a solute https://brainly.com/question/23946616
WILL GIVE BRAINLIEST
Which model provides more information-a chemical formula or a sketch of the molecule
like in the structural formula? Explain your reasoning.
Answers
Answer:
Explanation
:Both b/c a chemical formula tells you how many and a sketch formula shows how they are bonded together.
Molecular formulas show how many atoms of each element one molecule of a compound contains. Note: Ionic compounds are generally crystalline solids with high melting points. Other compounds, however, have very different properties.
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
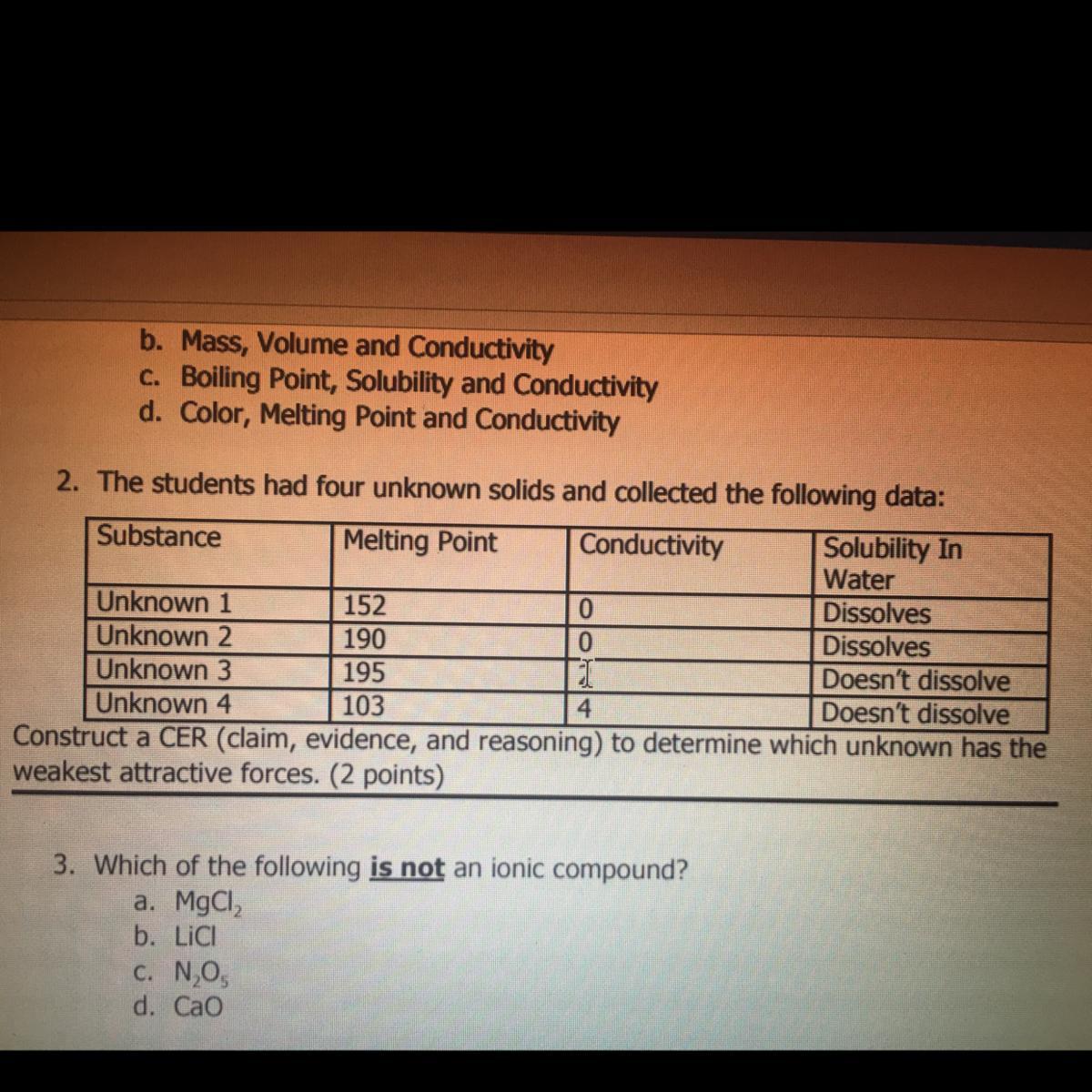
Answers
Answer:
Unknown 4
Explanation:
(10 points) What is the amount of radioactivity given off by a typical banana that contains 420 mg of Potassium, due to the presence of the natural isotope of 40 K? which has a half-life of 1.248 x 10
Answers
The amount of radioactivity given off by a typical banana that contains 420 mg of Potassium, due to the presence of the natural isotope of 40 K, is about 15 Bq.
The half-life of 40K is 1.248 x 10⁹ y, which is about 4.5 x 10¹⁶ s. The number of 40K atoms in 420 mg of Potassium is about 1.2 x 10²¹ atoms. The activity of 40K is given by the following equation:
A = λN
where A is the activity, λ is the decay constant, and N is the number of atoms. The decay constant for 40K is 6.30 x 10⁻¹¹ s⁻¹.
Plugging in the values, we get the following:
A = (6.30 x 10⁻¹¹ s⁻¹)(1.2 x 10²¹ atoms) = 7.5 x 10¹⁰ s⁻¹
The activity of 40K is measured in becquerels (Bq), where 1 Bq = 1 decay per second. So, the activity of 40K in a typical banana is about 15 Bq.
It is important to note that the amount of radioactivity given off by a banana is very small. The average person is exposed to about 300 mSv of radiation per year from natural sources, such as radon gas, cosmic rays, and the food we eat.
The amount of radiation given off by a banana is about 0.000005 mSv, which is about 0.0002% of the average annual exposure from natural sources. So, eating a banana will not increase your risk of radiation exposure.
To know more about the radioactivity refer here :
https://brainly.com/question/32007161#
#SPJ11
Complete question :
What is the amount of radioactivity given off by a typical banana that contains 420 mg of Potassium, due to the presence of the natural isotope of 40 K? which has a half-life of 1.248 x 10⁹ y and is 0.0117% of all Potassium. Atomic mass of K is 39.0983 g and A = 6.023 x 10 23 atoms
When Mg bonds with S, which of the following is true?a. Mg and S are in a "sea of electrons."b.Mg and S share two electrons.c. Mg gains two electrons, while S loses two electrons.d. Mg loses two electrons, while S gains two electrons.
Answers
Answer: Magnesium loses two electrons whilst sulfur gains tw
Which of the following is/are considered alcohols?
a CH3OH
b CH3CH2OH
C CH3CH2CH2OH
d All of the above
Answers
Answer: THE ANSWER IS D
Explanation:
an alcohol is a hydrocarbon chain with an hydroxyl group (OH)
A, B, AND C ARE ALL ALCOHOLS, SO THE ANSWER IS D
why there is no reaction when aluminium is added into cold dilute hydrochloric acid
Answers
Answer:
When aluminum is added to cold dilute hydrochloric acid, there is no reaction because aluminum is a highly reactive metal, but it is protected by a thin oxide layer on its surface. This oxide layer is not easily dissolved by dilute hydrochloric acid, so the aluminum does not react with the acid. In order to react with the acid, a stronger acid such as sulfuric acid or nitric acid is needed to dissolve the oxide layer. Additionally, a higher concentration of hydrochloric acid is also needed to react with aluminum.
Another possible reason is that Aluminium metal react with hydrochloric acid to produce hydrogen gas and aluminum chloride salt, but the reaction is relatively slow and requires heat to speed it up. In cold dilute hydrochloric acid, the reaction rate is too slow to observe any visible change.
Answer:
There is a leyer of aluminium that prevents nothing from happening
Explanation:
Keep in mind that this reaction will not take place as soon as you add the piece of aluminium to the hydrochloric acid solution. That happens because the piece of aluminium is protected by a layer of aluminium oxide, Al2O3 , the same layer that protects aluminium from reacting with water
1. which cell structure is represented by B?
a) vacuole
b) ribosome
c) cytoplasm
d) chloroplast
2. The nucleus contains molecules of A, which:
a) recycle waste products
b) remove water from the cell
c) store hereditary information
d) regulate the pH of cytoplasm

Answers
Answer:
1.B
2.C
Explanation:
Cell structure is represented by B is the ribosome.
The nucleus contains molecules of C, which store hereditary information.
What is a cell?Cells are the basic building blocks of all living things. The human body is composed of trillions of cells.
In a eukaryotic cell, ribosomes present in the cytoplasm synthesize proteins, which are required by the cell and the ribosomes present on the rough endoplasmic reticulum, synthesize secretory proteins.
Most of the enzymes are protein molecules and are synthesized on the ribosomes and the nucleus is contained with DNA that stores the hereditary information.
Hence, option B is correct for question 1 and option C is correct for question 2.
Learn more about the cell here:
https://brainly.com/question/5763151
#SPJ2
What do these two changes have in common?
baking a loaf of bread
cooking an egg
Select all that apply.
A.Both are only physical changes.
B.Both are caused by heating.
C.Both are chemical changes.
D.Both are caused by cooling.
Answers
Baking a loaf of bread and cooking an egg are only physical changes. Therefore, the correct option is option A.
A physical change gets a sort of change whereby the composition of matter is changed but not transformed. Although matter's size or shape may change, no chemical reaction takes place. Usually, physical changes are reversible. It should be noted that reversibility is not necessarily a need for a process to qualify as a physical change. Baking a loaf of bread and cooking an egg are only physical changes.
Therefore, the correct option is option A.
To know more about physical change, here:
https://brainly.com/question/17931044
#SPJ1
