Answers
Answer:
1.cation
2.anion
Explanation:
the plus is always cation and the negative is anion
Answer:
1. cation
2. anion
Explanation:
A positive ion is always cation and the negative ion is anion
Related Questions
What happens to the particles of a solid when the solid changes into a liquid? (5 points) a They increase in size. b They decrease in size. c The attractive force between them increases. d The attractive force between them decreases.
Answers
Although the volume that the particles take up generally increases when going from solid to liquid, particles themselves never change their size. It’s the attractive force that decreases and doesn’t pull the particles together as tightly when a solid changes to liquid.
Rank the following elements by decreasing metallic character: Cesium (C's), Bromine (Br), Osmium (Os), Tin (Sn)
Answers
Answer:
Cs, Os, Sn, Br
Explanation:
Metallic character increases to the left and down the periodic table.
Find the empirical formula for the compound if a sample contains 42.05g of nitrogen and 95.95g of oxygen.
Answers
Answer:
NO2 (nitrogen dioxide)
Explanation:
The empirical formula is the formula of the compound with its proportions of each element being in the simplest mole ratio.
That being said, first, we find the number of moles of each element.
n(N) = mass present/molar mass (m/M) = 42.05/14.01 (use a periodic table to locate the molar mass)
n(N) = 3.00143 mol
n(O) = m/M = 95.95/16.00 = 5.99688 mol
∴ the ratio of nitrogen to oxygen = 3.00143 : 5.99688
divide both sides by 3.00143, and we get 1 : 1.998,
which we can then round up to 1 : 2.
Hence, the empirical formula of the compound is \(NO_{2}\)
What volume is occupied by 0.101 mol of helium gas at a pressure of 0.91 atm and a temperature of 309 K
Answers
The volume occupied by 0.101 mol of helium gas at a pressure of 0.91 atm and a temperature of 309 K can be calculated using the ideal gas law equation.
The volume can be determined by rearranging the ideal gas law equation, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation to solve for V, we get V = (nRT)/P. Plugging in the given values, we have
V = (0.101 mol)(0.0821 L·atm/(mol·K))(309 K) / (0.91 atm).
By performing the calculation, the volume is found to be approximately 2.9 liters.
The calculation is based on the ideal gas law, which describes the behavior of ideal gases under certain conditions. In this case, the ideal gas law equation is used to find the volume of helium gas.
The equation relates the pressure, volume, temperature, and number of moles of a gas. By rearranging the equation and plugging in the given values, we can calculate the volume.
The volume is found to be 2.9 liters, indicating the amount of space occupied by 0.101 mol of helium gas at the given pressure and temperature.
It's important to note that this calculation assumes that helium behaves as an ideal gas, meaning that it follows the ideal gas law equation accurately under the given conditions.
for such more questions on volume
https://brainly.com/question/29796637
#SPJ8
HURRY PLS
Which of the following is a base-conjugate acid pair?
O H₂O and H3O+
O H₂O and H₂PO4
O H₂PO4 and HPO42-
O H₂PO4 and H3O+
Answers
Answer:
Option A
Explanation:
Remember than while finding conjugate aCid add a H+ to the base or compound
.Here
Water+H+
H_20+H+H_3O+Option A
A base conjugate acid pair is H₂O and H₃O⁺ and the correct option is option 1.
What are Conjugate acid base pair?
Conjugate acid-base pair refers to the pair of compounds that differ by a proton.
The pair of compounds which can mutually accept and donate hydrogen ions is called a conjugate acid-base pair.
A proton is added to obtain the conjugate acid and a proton is removed to get the conjugate base of the compound.
Therefore, A base conjugate acid pair is H₂O and H₃O⁺ and the correct option is option 1.
Learn more about Conjugate acid base pair, here:
https://brainly.com/question/13336099
#SPJ5
in what molecules does the presence of nonbinding electron pairs produce an effect on molecular shape
Answers
The presence of nonbonding electron pairs, also known as lone pairs or nonbonding electron domains, can have an effect on the shape of molecules. These lone pairs influence the molecular geometry by exerting electron repulsion and affecting the arrangement of atoms and bonding pairs.
Molecules that commonly exhibit the influence of nonbonding electron pairs on molecular shape include:
Water (H2O): In water, the two lone pairs of electrons on the oxygen atom affect the molecular shape, leading to a bent or V-shaped geometry.
Ammonia (NH3): Ammonia has one lone pair of electrons on the nitrogen atom, which leads to a pyramidal shape.
Nitrogen trifluoride (NF3): NF3 has one lone pair of electrons on the central nitrogen atom, resulting in a trigonal pyramidal shape.
Carbon dioxide (CO2): Although carbon dioxide does not possess any lone pairs on the carbon atom, the presence of two double bonds results in a linear molecular shape.
Sulfur hexafluoride (SF6): The six lone pairs of electrons on the sulfur atom in SF6 cause electronic repulsion, resulting in an octahedral shape.
These are just a few examples, but there are many molecules where nonbonding electron pairs influence the overall molecular shape. The presence and arrangement of these lone pairs affect the bond angles and distortions from ideal geometries in molecules, ultimately determining their three-dimensional shapes.
learn more about electron pairs here
https://brainly.com/question/29427403
#SPJ11
in the 13c nmr of benzil, which carbon is responsible for the resonance at 194.5 ppm? the other peaks are at 134.8, 132.9, 129.8 and 128.9 ppm. which carbon(s) are responsible for the resonances at 134.8 and 132.9 ppm? you do not need to assign each resonance, but identify which carbon(s) might give rise to these signals
Answers
In the 13C NMR spectrum of benzil, the carbon responsible for the resonance at 194.5 ppm is the carbonyl carbon of the ketone group, which is in the middle of the molecule.
The peaks at 134.8 and 132.9 ppm are likely due to the carbons in the aromatic ring adjacent to the carbonyl group.
The carbon directly adjacent to the carbonyl group (ortho position) usually appears at higher chemical shift values (around 135 ppm), while the next carbon (meta position) usually appears at slightly lower values (around 130 ppm).
Therefore, the peaks at 134.8 and 132.9 ppm likely correspond to the ortho and meta carbons, respectively.
The other peaks at 129.8 and 128.9 ppm are due to the carbons in the aromatic ring farthest from the carbonyl group, while the peak at 194.5 ppm is due to the carbonyl carbon in the ketone group.
to know more about 13C NMR spectrum refer here:
https://brainly.com/question/28260199#
#SPJ11
Testing precision and accuracy of scale, weigh block exactly 1.000g. these are measurements captured:
0.843 g
0.842 g
0.843 g
Is the scale precise, accurate, both, or neither?
Answers
we can conclude that the scale is precise but not accurate. The correct option is d.This means that the scale consistently gives the same measurements, but they are not accurate or close to the true value.
To understand whether the scale is precise, accurate, both, or neither, we need to define these terms. Precision refers to the consistency or reproducibility of measurements, while accuracy refers to how close the measured value is to the true or accepted value. In this case, the true value is 1.000g, and the measurements captured are 0.843 g, 0.842 g, and 0.843 g.Looking at these measurements, we can see that they are not accurate since none of them are close to 1.000g. However, we can also see that they are precise since they are all very similar to each other, with a difference of only 0.001g between the highest and lowest measurement.
Therefore, To improve accuracy, the scale may need to be recalibrated or replaced.
learn more about precise Refer: https://brainly.com/question/30641212
#SPJ11
complete question: Testing precision and accuracy of scale, weigh block exactly 1.000g. these are measurements captured:
a. 0.843 g
b. 0.842 g
c. 0.843 g
d. Is the scale precise, accurate, both, or neither?
A kicked soccer ball
eventually comes
to rest. What
force causes
this?
ce
Answers
Answer:
friction
Explanation:
jwjwkkskskaksksk
according to the information in the passage, in general, adding electrons to nonmetals is:
Answers
According to the information, in general, adding electrons to nonmetals is called reduction.
Nonmetals are elements in the periodic table that are generally not very reactive chemically.
In general, nonmetals have a low melting and boiling point, are poor conductors of heat and electricity, and have a tendency to gain electrons when they react with other elements.
Nonmetals typically form negative ions (anions) when they react with metals, which means they gain electrons.
This is called reduction, which occurs when electrons are added to an atom, reducing its oxidation state or oxidation number.
Additionally, nonmetals often form covalent bonds with other nonmetals by sharing electrons to form molecules.
This is in contrast to metals, which typically form ionic bonds by transferring electrons to form cations and anions.
To know more about electrons, visit:
https://brainly.com/question/12001116
#SPJ11
Ruth rode home at a constant speed for the first 10 minutes of the trip. What was her constant speed?
______ m/min
What was Ruth's average speed for the entire trip?
______ m/min
Ruth stopped to talk with another friend during the trip.
How far was she from home when she stopped?
______ m
How long was she stopped?
_____ min
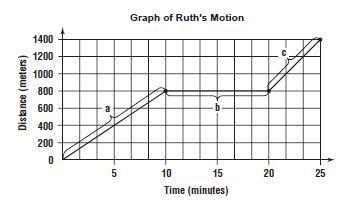
Answers
From the graph, the distance for the first 10 minutes is 600 meters.
10 minutes = 10 x 60 = 600 seconds
Ruth's speed for the first 10 minutes = distance/time
= 600/600 = 1 m/s
Average speed = total distance traveled/total time taken
Total distance = 1400 meters
Total time = 25 minutes = 25 x 60 = 1500 seconds
Average speed = 1400/1500 = 0.93 m/s
Ruth had traveled 800 meters before stopping to talk to her friend:
1400 - 800 = 600 meters.
Thus, she was 600 meters away from home when she stopped.
She stopped between 10 and 20 minutes:
20 - 10 = 10 minutes
Thus, she stopped to talk to her friend for 10 minutes.
More on velocity-time graphs can be found here: https://brainly.com/question/11290682
#SPJ1
When two or more different elements bond together, compounds are formed. The smallest parts of these compounds that retain their original properties are called molecules. All atoms contain negatively charged electrons that orbit around a positively charged nucleus. The nucleus contains positively charged ____________ and neutrally charged ____________ . It is the ____________ charged ____________ that give atoms properties that are favorable in forming chemical bonds.
Answers
Answer:
protons, neutrons, negatively, electrons
Explanation:
protons are positive, neutrons are neutral. the entire basis of bonding is chemistry is the distribution of electrons, which are negatively charged particles surrounding the nucleus.
add (from buret) slowly, with constant stirring, calculated amount of 0.2 f agno3. add 10% excess. [instructor will provide the 0.2 m agno3.]
Answers
To perform the procedure, slowly add the calculated amount of 0.2 F AgNO3 from a buret while maintaining constant stirring. Additionally, incorporate a 10% excess of AgNO3 as directed by the instructor.
In this procedure, the objective is to add a specific amount of 0.2 F AgNO3 solution with constant stirring. The use of a buret allows for precise control over the volume being added. By adding the solution slowly, the reaction can be monitored and controlled more effectively.
Furthermore, it is mentioned that a 10% excess of AgNO3 should be added. This means that an additional 10% of the calculated amount of AgNO3 should be incorporated. The purpose of this excess is to ensure that all the reactants are fully consumed, promoting a complete reaction and maximizing the desired outcome.
The instructor will provide the 0.2 M AgNO3 solution, which is a solution with a known concentration. This concentration allows for accurate calculations of the required volume to achieve the desired amount of AgNO3.
Learn more about excess of AgNO3
brainly.com/question/10052452
#SPJ11
the largest group of the ferrous based metals includes the
Answers
The largest group of ferrous-based metals includes the steel and iron family.
Steel is a composite material composed of iron and carbon, with the carbon content varying within a range of up to 2 percent. These metals are distinguished by their iron composition and their magnetic characteristics. Due to their strength, longevity, and cost-effectiveness, they find extensive applications in construction, transportation, and various industries.
The primary constituent of steel is iron, a metal that, in its pure form, is only slightly harder than copper. Unless considering highly exceptional scenarios, solid iron, like other metals, is polycrystalline, meaning it is composed of multiple crystals that interconnect along their boundaries.
A crystal refers to a precisely organized configuration of atoms that can be visualized as spheres in contact with one another. These atoms are arranged in planes known as lattices, which intersect each other in specific patterns. In the case of iron, the lattice arrangement can be most effectively envisioned as a unit cube containing eight iron atoms positioned at its corners.
To learn more about ferrous metal visit:
https://brainly.com/question/9347985
#SPJ11
Why do we standardize the naoh solution which we made by dissolving a measured mass of solid NaOH?
Answers
Standardizing a sodium hydroxide solution is necessary because NaOH reacts with atmospheric carbon dioxide to form sodium carbonate and water, reducing the accuracy of its concentration.
We standardize a sodium hydroxide (NaOH) solution, which means we determine its exact concentration, because NaOH is a strong base that reacts with atmospheric carbon dioxide (CO₂) to form sodium carbonate (Na₂CO₃) and water (H₂O):
2NaOH (aq) + CO₂ (g) → Na₂CO₃ (aq) + H₂O (l)
This reaction reduces the concentration of NaOH in the solution, making it less accurate for titrations or other chemical analyses. Standardizing the NaOH solution involves titrating it with a known concentration of an acid, such as hydrochloric acid (HCl), using an appropriate indicator to determine the exact concentration of NaOH.
During the titration, the acid and base react in a 1:1 ratio according to the balanced chemical equation:
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
The endpoint of the titration is reached when all the HCl has reacted with the NaOH, and the solution becomes neutral. An indicator, such as phenolphthalein, is used to signal the endpoint of the titration, where the indicator changes color from pink to colorless. The volume of acid required to reach the endpoint is measured, and the concentration of NaOH is calculated using stoichiometry.
Standardizing the NaOH solution ensures that its concentration is accurately known, allowing it to be used in subsequent chemical reactions or analyses. It is important to standardize the NaOH solution periodically, as the concentration can change over time due to factors such as atmospheric carbon dioxide absorption, water absorption, or contamination.
To know more about solution please refer: https://brainly.com/question/30665317
#SPJ4
If a neutral atom of Bromine (Br) was to gain another electron, it would have the same electron configuration as which element?
Answers
Answer:
Krypton (Kr)
Explanation:
We'll begin by writing the electronic configuration of bromine. This is illustrated below:
Br (35) => [Ar]4s² 3d¹⁰ 4p⁵
Since the bromine atom gains an extra electron, the number of electrons in the bromide ion, Br¯ becomes 36. Hence the electronic configuration of the bromide ion, Br¯ can be written as follow:
Br¯ (36) => [Ar]4s² 3d¹⁰ 4p⁶
Now, comparing the number of electrons in the bromide ion, Br¯ with those in the periodic table, it is evident that the bromide ion, Br¯ has the same number of electrons with krypton, Kr. Hence, the bromide ion, Br¯ and the krypton, Kr has the same electronic configuration i.e
Br¯ (36) => [Ar]4s² 3d¹⁰ 4p⁶
Kr (36) => [Ar] 4s² 3d¹⁰ 4p⁶
A sample of helium gas has a volume of 6.50l at a pressure of 845 mmhg and a temperature of 25oc. what is the pressure of the gas in atm when the volume and temperature of the gas sample are changed to 1850 ml and 325 k
Answers
Answer:
4.25atm
Explanation:
\(\frac{P1V1}{T1}\\\)=\(\frac{P2V2}{T2}\)
where ,
p1 =845/760=1.11atm
v1=6.50l
t1=25c +273k=298k
p2=?
v2=1850/1000=1.85l
t2=325k
so answer is 4.25 atm
Part B
Now decide how many different combinations of baking soda and vinegar you will try, The number of combinations must be three or more.
Answers
The many different combinations of baking soda and vinegar to try would be:
50 mL vinegar and 10 g baking soda50 ml vinegar and 5 g baking soda50 mL vinegar and 15 g of baking soda.What is the reaction of vinegar and baking soda?The reaction between vinegar and baking soda is essentially the reaction between sodium bicarbonate and acetic acid.
The equation of the reaction is given below:
Sodium carbonate + acetic acid ---> Sodium acetate + water + carbon dioxide.
It is clear that carbon dioxide gas was produced when the solid baking soda was mixed with the liquid vinegar because bubbles started to appear in the mixture as it foamed.
Learn more about vinegar and baking soda at:
#SPJ1
How many moles of molecules and atoms are in 3.07g sample of SO3
Molecules____
Atoms_______
Answers
Answer:
ammonia molecules. Similar factors may be derived for any pair of substances in any chemical equation. Example 4.8. Moles of Reactant Required in a Reaction.
Explanation:
When is the change of in enthalpy when 77. 2 grams of steam at 100c is converted liquid water at the same temperature and temperature?
Answers
The change in enthalpy, or heat of vaporization, when 77.2 grams of steam at 100°C is converted to liquid water at the same temperature is approximately 40.7 kJ/mol.
This value represents the amount of energy that must be removed from the steam to condense it into liquid water at 100°C. It is important to note that this value may vary slightly depending on the exact pressure and other conditions of the system.
The change in enthalpy, also known as the enthalpy of vaporization, occurs when steam is converted to liquid water at the same temperature. For this process, 77.2 grams of steam at 100°C is converted to liquid water at 100°C.
To calculate the change in enthalpy, we can use the formula:
ΔH = m × ΔHvap
where ΔH is the change in enthalpy, m is the mass of the steam (77.2 grams), and ΔHvap is the enthalpy of vaporization of water (approximately 40.7 kJ/mol at 100°C).
First, we need to convert the mass of steam to moles using the molar mass of water (18.015 g/mol):
moles of steam = (77.2 g) / (18.015 g/mol) ≈ 4.29 moles
Now we can calculate the change in enthalpy:
ΔH = (4.29 moles) × (40.7 kJ/mol) ≈ 174.6 kJ
So, the change in enthalpy when 77.2 grams of steam at 100°C is converted to liquid water at the same temperature is approximately 174.6 kJ.
Visit here to learn more about enthalpy : https://brainly.com/question/29145818
#SPJ11
10. You can protect yourself from radiation by
going up to a higher elevation
increasing the time you are exposed
increasing your water intake
getting further away from the radiation source
Answers
Answer:
You can protect yourself from radiation by getting further away from the radiation source .
Explanation: The further you are from the radiation source, the less it effects you and the safer you are.
what value for a substance can be determined by plotting its absorption spectrum?
Answers
The absorption spectrum is used to determine the wavelength of a substance.
Electromagnetic radiation consists of different radiations of different frequency. The wavelengths of these radiations also differ from each other. When white light is allowed to pass through a medium it gets split into different radiations having different frequencies and wavelengths. These radiations interact with the molecules and matter present in the air and these radiations absorb energy and reach a higher energy level. If the higher energy levels are unstable, then they emit energy to reach to their ground state. This series of emissions and absorptions form a spectra known as the Absorption Spectra or Absorption Spectrum.
Learn more about Absorption Spectrum at:
brainly.com/question/10252035
#SPJ4
why should bunsen burners not be used when heating organic materials?
Answers
Answer:
Many organic compounds are very flammable.
Explanation:
Because we do not want to inadvertently ignite chemicals and cause a fire or explosion. Most organic chemicals are incompatible with inorganic chemicals, and certain mixtures can cause violent reactions.
Mass is a measure of how much matter something has.
False
True
Answers
Answer:
true
Explanation:
Which best represents a homogeneous mixture of an element and a compound.
Answers
Answer:
The composition of air this is because it vmade up of oxygen, nitrogen, Nobel gages and Carbon dioxide
how are subtances dissolved ?
Answers
Answer: A solute dissolves because its particles interact with the particles of a solvent. Anything that allows more solvent to touch more solute will cause a solute to dissolve more quickly. Small pieces of a substance dissolve faster than large pieces.
how much energy has your body used, in joules, if your health device indicates that 450 calories were burned during your workout
Answers
Answer:
Total energy consumed = 1,882.8 joules
Explanation:
Given:
Calories burned = 450 calories
Find:
Total energy consumed
Computation:
1 calorie = 4.184 joules
So,
450 calories = 4.184 × 450
450 calories = 1,882.8 joules
Total energy consumed = 1,882.8 joules
What would happen if you mixed aqueous magnesium bromide with aqueous sodium phosphate?
Answers
Answer:
When heated, solid calcium chlorate decomposes into calcium chloride solid, releasing oxygen gas. (c) Solutions of barium bromide and sodium phosphate combine to ... iodide are mixed together, forming solid silver iodide and aqueous .
is 3NaOH + H3PO4--->Na3PO4 + 3H2O balanced?
Answers
Answer:
Yes
Explanation:
3NaOH + H3PO4
3Na + 3O + 3H + 3H + P + 4O
3Na + 7O + 6H + P
Na3PO4 + 3H2O
3Na + P + 4O + 6H + 3O
3Na + 7O + 6H + P
PREPARATION OF BASES
Answers
The preparation of bases involves several methods that are used to create substances with basic or alkaline properties are Reaction of metal with water, Reaction of metal oxide with water, Neutralization reaction, Ammonia gas dissolving in water and Partial neutralization of a strong base with a weak acid.
Reaction of metal with water: Certain metals, such as sodium or potassium, react with water to form hydroxides. For example, sodium reacts with water to produce sodium hydroxide (NaOH).
Reaction of metal oxide with water: Metal oxides, such as calcium oxide (CaO) or magnesium oxide (MgO), can be added to water to form metal hydroxides. This process is known as hydration. For instance, when calcium oxide reacts with water, it forms calcium hydroxide (Ca(OH)2).
Neutralization reaction: Bases can be prepared by neutralizing an acid with an appropriate alkaline substance. This involves combining an acid with a base to form water and a salt. For example, mixing hydrochloric acid (HCl) with sodium hydroxide (NaOH) results in the formation of water and sodium chloride (NaCl).
Ammonia gas dissolving in water: Ammonia gas (NH3) can dissolve in water to form ammonium hydroxide (NH4OH), which is a weak base.
Partial neutralization of a strong base with a weak acid: Mixing a strong base, such as sodium hydroxide (NaOH), with a weak acid, like acetic acid (CH3COOH), results in the formation of a base with a lesser degree of alkalinity.
These methods are utilized in laboratories, industries, and various applications where bases are required, such as in the production of cleaning agents, pharmaceuticals, and chemical reactions. Each method has its own advantages and specific applications depending on the desired base and its properties.
The question was incomplete. find the full content below:
What are the various methods involved in the preparation of bases?
Know more about Neutralization Reaction here:
https://brainly.com/question/23008798
#SPJ8
