Answers
Answer:
That is the Atomic mass
Explanation:
The element symbol is S, Element name is sulfur, and
Atomic number is 16
Related Questions
A gas at constant temperature has a pressure of 404.6 kPa with a volume of 12 ml. If the volume changes to 43ml, what is the new pressure
Answers
Answer:
The answer is
112.912 kPaExplanation:
The new pressure can be found by using the formula for Boyle's law which is
\(P_1V_1 = P_2V_2\)
Since we are finding the new pressure
\(P_2 = \frac{P_1V_1}{V_2} \\\)
404.6 kPa = 404600 Pa
From the question we have
\(P_2 = \frac{404600 \times 12}{43} = \frac{4855200}{43} \\ = 112911.6279... \\ = 112912\)
We have the final answer as
112.912 kPaHope this helps you
Why is k+ much more stable than k2+?
Answers
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
How many photons are contained in a flash of green light (525 nm) that
contains 189 kJ of total energy?
Answers
The flash of green light 525 nm that contains 189kJ of total energy is 2.39 ×10^29 photons.
The formula below gives the energy EE of a single photon of wavelength lambda.
E = hc/λ, where c is the speed of light and h is the plank constant, with a value of 6.62607015 ×10^34.
To determine the overall number of photons n in a green light flash (525 mm) with a total energy of 189 kJ.
We'll apply the following formula.
n= Et/ E
When we enter the given values into the formula, we see that n= Et/E.
= \(\frac{189 *10^3}{6.62607015 *10^−34 × 3*10^8}/ 525 *10^-3\)
Therefore, there are 2.39 ×10^29 photons in a green light with 189 kJ of energy.
For more information on photons kindly visit to
https://brainly.com/question/20912241
#SPJ1
How many moles are in 15 grams of Li?
Answers
Which of the following technique is used to purify the impurities that are not very different in chemical properties of element? [a] Gas chromatography [b] Column chromatography [c] TLC [d] HPLC
Answers
Answer:
Explanation: Liquid Chromatography
I'm sorry if i'm wrong
Which of the following correctly describes a mixture?
Answers
A mixture can be defined as a physical blend of two or more substances that are not chemically combined.
They retain their own properties and can be separated by physical means like filtration, distillation, evaporation, or magnetism. The various types of mixtures include homogeneous mixtures, heterogeneous mixtures, and colloids.Homogeneous mixtures, also known as solutions, are uniform mixtures where the composition is the same throughout. They are not visibly different and consist of a solute (the substance being dissolved) and a solvent (the substance doing the dissolving). For example, salt water is a homogeneous mixture because the salt is dissolved uniformly throughout the water.Heterogeneous mixtures are non-uniform mixtures that consist of two or more phases, each with its own distinct properties. They can be seen with the eye, and the different components can be separated using physical means. An example of a heterogeneous mixture is oil and water. They can be mixed together, but they will eventually separate.Colloids are mixtures where the particle size is intermediate between that of a solution and a suspension. The particles are small enough to not be visible to the eye, but they are large enough to scatter light. Milk is an example of a colloid because it appears homogeneous but is actually made up of small particles of fat and protein dispersed throughout the liquid.In conclusion, a mixture is a physical blend of two or more substances that are not chemically combined. They can be separated by physical means and consist of homogeneous mixtures, heterogeneous mixtures, and colloids.
for such more questions on substances
https://brainly.com/question/29108029
#SPJ8
This time, include both the coefficient and exponent. Express 0.00212 in scientific notation.
[?] * times 10^[?]
Enter the coefficient in the green box and the exponent in the yellow box.
Coefficient (green) Exponent (yellow)
_______________ _____________ Enter
![This time, include both the coefficient and exponent. Express 0.00212 in scientific notation.[?] * times](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/lnMi2a3nGuhR8UXZWuiEWNQeM7a27uTR.jpeg)
Answers
Answer: 212
Explanation:
explain the relationship (linear or exponential) between rate and concentration including what order the iodate ion would be in.
CONCENTRATIONS
EXP. 1: 0.020
EXP 2: 0.019
EXP 3: 0.017
EXP 4: 0.016
EXP 5: 0.014
EXP 6: 0.013
EXP 7: 0.011
EXP 8: 0.01
EXP 9: 8.6x10^-3
EXP 10: 7.1x10^-3
EXP 11: 5.7x10^-3
EXP 12: 4.3x10^-3
RATE (s^-1):
EXP 1: 0.283
EXP 2: 0.1972
EXP 3: 0.2353
EXP 4: 0.2033
EXP 5: 0.1701
EXP 6: 0.133
EXP 7: 0.10
EXP 8: 0.1234
EXP 9: 0.077
EXP 10: 0.07380
EXP 11: 0.05102
EXP 12: 0.03883
By looking at the reaction mechanism, propose a Rate Law (WITHOUT the value of K). Explain the exponents for each reactant. Also, how does the rate law proposed compared to the relationship between rate and iodate concentration observed in the Rate law question?
Discuss, with respect to collision theory, the changes in the rates result from the changing concentrations of the iodate ion. What would you predict if we repeated these reactions at higher temperatures? Explain using collision theory.

Answers
Based on the given data, the relationship between rate and concentration is exponential.
A proposed rate law for the reaction based on the given data is:
Rate = k[IO3⁻]²[H+]What is the collision theory?Collision theory suggests that the rate of a chemical reaction is proportional to the frequency and energy of collisions between the reactant molecules.
As the concentration of iodate ions decreases, the frequency of collisions between reactant molecules decreases, which leads to a decrease in the rate of the reaction.
At higher temperatures, the kinetic energy of the reactant molecules increases, which increases the frequency and energy of collisions between reactant molecules.
Learn more about collision theory at: https://brainly.com/question/20628781
#SPJ1
I am a teacher in a college in a large city in China. One day, some students asked me to join
them for dinner. They said they wanted to take me to a very special restaurant that had
just opened. This new place served American food. The students were proud that their city
had a restaurant that served fine American food. I was quite surprised when we got to the
restaurant. My students had taken me to a very well-known fast food restaurant!
1.
In the paragraph above, the author's purpose is to
А. inform.
B
entertain
share personal experiences
C
D
persuade
Answers
Answer:
c or b
Explanation:
the story is entertaining and it shares the teachers personal experiences with the teachers students
A solid keeps its shape due to which of the following factors? (3 points)
а
Space between particles
Attractive forces between particles
b
Ос
The type of element in the solid
Od
The container it is placed in
Answers
Answer: Attractive forces between particels
Explanation:
If a photon of monochromatic light has a wavelength of 94 nm, what is its frequency (in units of 1/s)?
What is the energy of this photon (in Joules)?
What is the energy of 1 mole of these photons (in kJ/mol)?
Answers
The energy of the wave is 2.11 * 10^-18 J while the energy per mole is 3.5 * 10^-42 J/mol.
What is the energy?We know that the energy of the photon is dependent on the wavelength of the light as we know. In this case, we can see that the wavelength of the light is obtained as 94 nm. We shall now proceed to find the parameters as required in the question.
We have the following;
E = hc/λ
E = energy of the radiation
h = Plank's constant
c = speed of light
λ = wavelength
Then we have;
E = 6.6 * 10^-34 * 3* 10^8/94 * 10^-9
E = 2.11 * 10^-18 J
The energy per mole is obtained from;
2.11 * 10^-18 J/6.02 * 10^23
= 3.5 * 10^-42 J/mol
Learn more about wavelength:https://brainly.com/question/13533093
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Calculate the amount of heat needed to boil 120.g of acetic acid (HCH3CO2), beginning from a temperature of 16.7°C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
The energy required to bring 120 g of acetic acid (HCH3CO2) to a boil at a starting temperature of 16.7 °C is 72.2116 kJ.
Given that ;
mass of acetic acid = 120.0 g
initial temperatureT₁ = 16.7 °C = (16.7 + 273.15 ) K = 298.85 K
standard molar mass of acetic acid = 60.052 g/mol
The number of moles of acetic acid can therefore be calculated as follows: number of moles of acetic acid = mass of acetic acid/molar mass of acetic acid
number of moles of acetic acid = 120.0 g/ 60.052 g/mol
number of moles of acetic acid = 1.998 moles
For acetic acid:
standard boiling point T₂ = 118.1 °C = ( 118.1 + 273.15 ) K = 391.25 K
enthalpy of vaporization of acetic acid ΔH\(_{vap}\) = 23.7 kJ/mol
heat capacity of acetic acid c = 2.043 J/g.K
change in temperature Δ T = T₂ - T₁
Δ T = (391.25 - 289.85)K
Δ T = 101.4 K
The heat required to raise the liquid acetic acid's temperature from 16.7°C to its boiling point is;
q = mcΔT
From our values above;
q = 120 g × 2.043 J/g.K × 101.4 K
q = 24859.2 J
q = 24859 /1000 kJ
q = 24.859 kJ
We already determined that we have 1.998 moles of acetic acid;
Thus;
the needed amount of heat = Δ\(_{vap}\)*number of moles
The needed amount of heat = 47.3526 kJ
Hence;
The total amount of heat needed = 24.859 kJ + 47.3526 kJ
The total amount of heat needed = 72.2116 kJ
Learn more about acetic acid here:
https://brainly.com/question/24194581
#SPJ4
Differences between voltage, current and resistance?
Answers
Answer:
Voltage is the measure of electric potential energy per unit charge, current is the flow of electric charge through a circuit, and resistance is the property of a material that opposes the flow of electric current.
Ohm's Law relates these three concepts by stating that current is directly proportional to voltage and inversely proportional to resistance.
Hope this helps!
If you have access to stock solutions of 1.00 M H3PO4, 1.00 M of HCl, and 1.00 M NaOH solution, (and distilled water of course), what volumes of each would you mix before diluting to a final volume of 2.00 L to prepare 2.00 L of pH 7.40 buffer with a final total concentration of 50 mM of phosphorous contains species (e.g. so that [H3PO4] [H2PO4 - ] [HPO4 2- ] [PO4 3- ]
Answers
Answer:
0.10L of 1.00M of H₃PO₄ and 0.1613L of 1.00M NaOH
Explanation:
The pKa's of phosphoric acid are:
H₃PO₄/H₂PO₄⁻ = 2.1
H₂PO₄⁻/HPO₄²⁻ = 7.2
HPO₄²⁻/PO₄³⁻ = 12.0
To make a buffer with pH 9.40 we need to convert all H₃PO₄ to H₂PO₄⁻ and an amount of H₂PO₄⁻ to HPO₄²⁻
To have a 50mM solution of phosphoures we need:
2L * (0.050mol / L) = 0.10 moles of H₃PO₄
0.10 mol * (1L / mol) = 0.10L of 1.00M of H3PO4
To convert the H₃PO₄ to H₂PO₄⁻ and to HPO₄²⁻ must be added NaOH, thus:
H₃PO₄ + NaOH → H₂PO₄⁻ + H₂O + Na⁺
H₂PO₄⁻ + NaOH → HPO₄²⁻ + H₂O + Na⁺
Using H-H equation we can find the amount of NaOH added:
pH = pKa + log [A⁻] / [HA] (1)
Where [A-] is conjugate base, HPO₄²⁻ and [HA] is weak acid, H₂PO₄⁻
pH = 7.40
pKa = 7.20
[A-] + [HA] = 0.10moles (2)
Replacing (2) in (1):
7.40 = 7.20 + log 0.10mol - [HA] / [HA]
0.2 = log 0.10mol - [HA] / [HA]
1.5849 = 0.10mol - [HA] / [HA]
1.5849 [HA] = 0.10mol - [HA]
2.5849[HA] = 0.10mol
[HA] = 0.0387 moles = H₂PO₄⁻ moles
That means moles of HPO₄²⁻ are 0.10mol - 0.0387moles = 0.0613 moles
The moles of NaOH needed to convert all H₃PO₄ in H₂PO₄⁻ are 0.10 moles
And moles needed to obtain 0.0613 moles of HPO₄²⁻ are 0.0613 moles
Total moles of NaOH are 0.1613moles * (1L / 1mol) = 0.1613L of 1.00M NaOH
Then, you need to dilute both solutions to 2.00L with distilled water.
A galvanic cell was constructed using cadmium and zine half cells. The voltage produced was measured to be 0.31 V at 25 °C; however, the standard cell potential for this reaction is 0.36 V. Which of the following statements provides the most likely source of this deviation?
Answers
\([Zn^+^2] [Sn^+^2]\) is the most plausible source of this deviation. This is because neither the reaction quotient Q nor the conventional equilibrium constant K are equivalent under the present conditions of the reaction. Standard cell potential (E°), real cell potential (E), gas constant (R), temperature (T), Faraday constant (F), and reaction quotient (Q) are all related by the following formula:
E = E° - (RT/nF) ln Q
Q must be greater than K because E is greater than E°. The reaction quotient Q can be calculated by:
Q = [Sn]/[Zn + 2]
The measured voltage will be greater than the normal cell potential if [Zn+2] [Sn+2] and Q > K.
So, the correct option is C.
Learn kore about Measured voltage here:
https://brainly.com/question/29357294
#SPJ1
Your question is incomplete, most probably the complete question is:
Sn2+ (aq) + Zn (s) → Sn (s) + Zn+2 (aq)
A galvanic cell was constructed using tin and zinc half cells. The voltage produced was measured to be 0.66 V at 25°C. However, the standard cell potential for this reaction is 0.62 V. Which of the following statements is the most likely source of this deviation?
(A) The salt bridge contained Na2SO4.
(B) SO42 was the anion at the anode and Cl was the anion at the cathode. (C) [Zn+2] < [Sn+2]
(D) The anode has a larger mass than the cathode.
(E) The volume of the Sn2 solution is greater than the volume of the Zn+2 solution.
Which part of the brain is affected by a smell, a song, or a photograph?
A. the primitive brain
B. the right brain
C. the outer brain
D. the emotional brain
Answers
The right brain part of the brain is affected by a smell, a song, or a photograph.
What is Right Brain?The right brain is more intuitive and visually oriented. It is occasionally referred to as the analog brain. It thinks less logically and with more creativity. According to out-of-date research by Sperry, the right brain aids in creativity.
Which part of brain deals with photograph?Posterior parietal cortex of the parietal lobe of the brain mainly deals with the photographs.
Which part of brain deals with Smell?The olfactory bulb, a structure in the front of the brain, processes smells before sending data to other regions of the body's central nervous system.
To know more about smelling part of brain visit
https://brainly.com/question/3655509
#SPJ1
The right brain part of the brain is affected by a smell, a song, or a photograph.
What is Right Brain?
The right brain is more intuitive and visually oriented. It is occasionally referred to as the analog brain. It thinks less logically and with more creativity. According to out-of-date research by Sperry, the right brain aids in creativity.
Which part of brain deals with photograph?
Posterior parietal cortex of the parietal lobe of the brain mainly deals with the photographs.
Which part of brain deals with Smell?
The olfactory bulb, a structure in the front of the brain, processes smells before sending data to other regions of the body's central nervous system.
In the Hall-Heroult process, a large electric current is passed through a solution of aluminum oxide Al2O3 dissolved in molten cryolite Na3AlF6, resulting in the reduction of the Al2O3 to pure aluminum. Suppose a current of 100.A is passed through a Hall-Heroult cell for 41.0 seconds. Calculate the mass of pure aluminum produced. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
Answer:
0.382g
Explanation:
Step 1: Write the reduction half-reaction
Al³⁺(aq) + 3 e⁻ ⇒ Al(s)
Step 2: Calculate the mass of Al produced when a current of 100. A passes through the cell for 41.0 s
We will use the following relationships.
1 A = 1 C/s1 mole of electrons has a charge of 96486 C (Faraday's constant)1 mole of Al is produced when 3 moles of electrons pass through the cell.The molar mass of Al is 26.98 g/mol.The mass of Al produced is:
\(41.0s \times \frac{100C}{s} \times \frac{1mole^{-} }{96486C} \times \frac{1molAl}{3mole^{-} } \times \frac{26.98gAl}{1molAl} = 0.382gAl\)
write on nitrogen and state it uses and explain it process and draw a nitrogen cycle and explain it test
Answers
Nitrogen is a chemical element with the symbol N and atomic number 7. It is a nonmetal and makes up about 78% of the Earth's atmosphere. Nitrogen is essential for all forms of life on Earth as it is a critical component of DNA, RNA, and proteins.
Uses:
Nitrogen has many uses in industry, agriculture, and medicine. In the industrial sector, nitrogen is used in the production of ammonia, which is used to make fertilizers, explosives, and other chemicals. Nitrogen gas is also used to create a controlled atmosphere in food storage and packaging, to prevent spoilage and preserve freshness. In the medical field, nitrogen is used to preserve blood, tissues, and other biological samples.
Process:
Nitrogen gas is produced through a process called fractional distillation, which separates the gases in the air based on their boiling points. Nitrogen gas is obtained by cooling air until it becomes a liquid, and then slowly warming it up again. Nitrogen gas boils at a lower temperature than oxygen gas, so it is collected as a gas as the temperature rises.
Nitrogen Cycle:
The nitrogen cycle is the process by which nitrogen is converted between its various chemical forms in the environment. The nitrogen cycle is important because it allows nitrogen to be used by living organisms, and it helps to regulate the levels of nitrogen in the atmosphere and in the soil.
The nitrogen cycle consists of four main stages: nitrogen fixation, nitrification, denitrification, and ammonification. Nitrogen fixation is the process by which nitrogen gas from the atmosphere is converted into a form that can be used by living organisms, such as ammonia or nitrate. This process is carried out by certain types of bacteria that live in the soil or in the roots of plants.
Nitrification is the process by which ammonia is converted into nitrate by other types of bacteria. This nitrate can then be absorbed by plants and used to make proteins and other essential molecules.
Denitrification is the process by which nitrate is converted back into nitrogen gas, which is released back into the atmosphere. This process is carried out by certain types of bacteria that live in oxygen-poor environments, such as wetlands or soils that are waterlogged.
Ammonification is the process by which organic nitrogen compounds, such as proteins or amino acids, are broken down into ammonia by bacteria and fungi. This ammonia can then be used by other organisms in the nitrogen cycle.
Testing:
The most common test for the presence of nitrogen is the Kjeldahl method, which is used to determine the amount of nitrogen in organic compounds. The Kjeldahl method involves heating a sample of the organic compound with sulfuric acid, which converts the nitrogen into ammonia. The ammonia is then distilled off and collected in a solution of boric acid, which forms a compound called ammonium borate. The amount of nitrogen in the original sample can then be determined by measuring the amount of ammonium borate that is produced.
B and I chemical formula?
Answers
How many moles of atoms are in each elemental sample?
(a) 4.6 X 1024 Pb atoms
(b) 2.87 × 10- He atoms
(c) 7.91 × 102 K atoms
(d) 4.41 × 10- Ca atoms
Answers
(a) For 4.6 x 10^24 Pb atoms, the number of moles is: 4.6 x 10^24 Pb atoms / 6.022 x 10^23 atoms/mol = 0.764 mol Pb
(b) For 2.87 x 10^-2 He atoms, the number of moles is: 2.87 x 10^-2 He atoms / 6.022 x 10^23 atoms/mol = 4.77 x 10^-26 mol He
(c) For 7.91 x 10^2 K atoms, the number of moles is: 7.91 x 10^2 K atoms / 6.022 x 10^23 atoms/mol = 1.31 x 10^-21 mol K
(d) For 4.41 x 10^-2 Ca atoms, the number of moles is: 4.41 x 10^-2 Ca atoms / 6.022 x 10^23 atoms/mol = 7.30 x 10^-26 mol Ca
i need to know the measurements of this to the appropriate amount of significant figures

Answers
Answer:
[See Below]
Explanation:
23 or 23.5 ml.
What is the electron domain geometry around N in N2CL4
Answers
Answer:
trigonal bipyramidal.
Which of the following relationships between
the pressure P, the volume-V and the
e temperature T, represents an ideal gas
behaviour?
A.paVT
B.p TaT/V
C. pTaVet
D.pval/T
E. paV/T
Answers
Out of the options given, the expression that represents the ideal gas behavior is:
E. P × V / TWhat is ideal gas equation?The ideal gas law is expressed by the formula PV = nRT,
where
P is the pressure,
V is the volume,
n is the number of moles of gas,
R is the gas constant, and
T is the temperature.
By rearranging this equation, we can derive different expressions for the ideal gas behavior in terms of the pressure, volume, and temperature.
This can be obtained by rearranging the ideal gas law equation as follows:
PV = nRT
Dividing both sides by nT, we get:
P × V / (nT) = R
Since R is a constant for a given gas, the left-hand side of the equation must also be constant for an ideal gas. Thus, the expression P × V / T represents the ideal gas behavior.
Learn more about ideal gas at
https://brainly.com/question/27870704
#SPJ1
A basketball with a mass of 0.60 kg is accelerated with a force of 10.8 N. If resisting forces
are ignored, what is the acceleration of the basketball to the nearest m/s 2
Answers
Answer:
18 m/s²Explanation:
The acceleration of an object given it's mass and the force acting on it can be found by using the formula
\(a = \frac{f}{m} \\ \)
f is the force
m is the mass
From the question we have
\(a = \frac{10.8}{0.6} \\ \)
We have the final answer as
18 m/s²Hope this helps you
Which type of electromagnetic wave has the most energy?
X-rays
X-rays
gamma rays
gamma rays
radio waves
radio waves
visible light
Answers
Answer:
gamma rays please give brainliestDirections: The picture below shows a model of Earth's layers. Use the picture to answer
any questions that follow.
Layer 5
Layer 4
Layer 3
Layer 2
Layer 1
1. Which layers compose the lithosphere?
A layers 6 and 4
B layers 4 and 3
a layers 3 and 2
D layers 2 and 1
Answers
Answer:
hfghfhjcbnggf
Explanation:
layer1
layer 2
Liquid water - heat =
Pls help now!!!
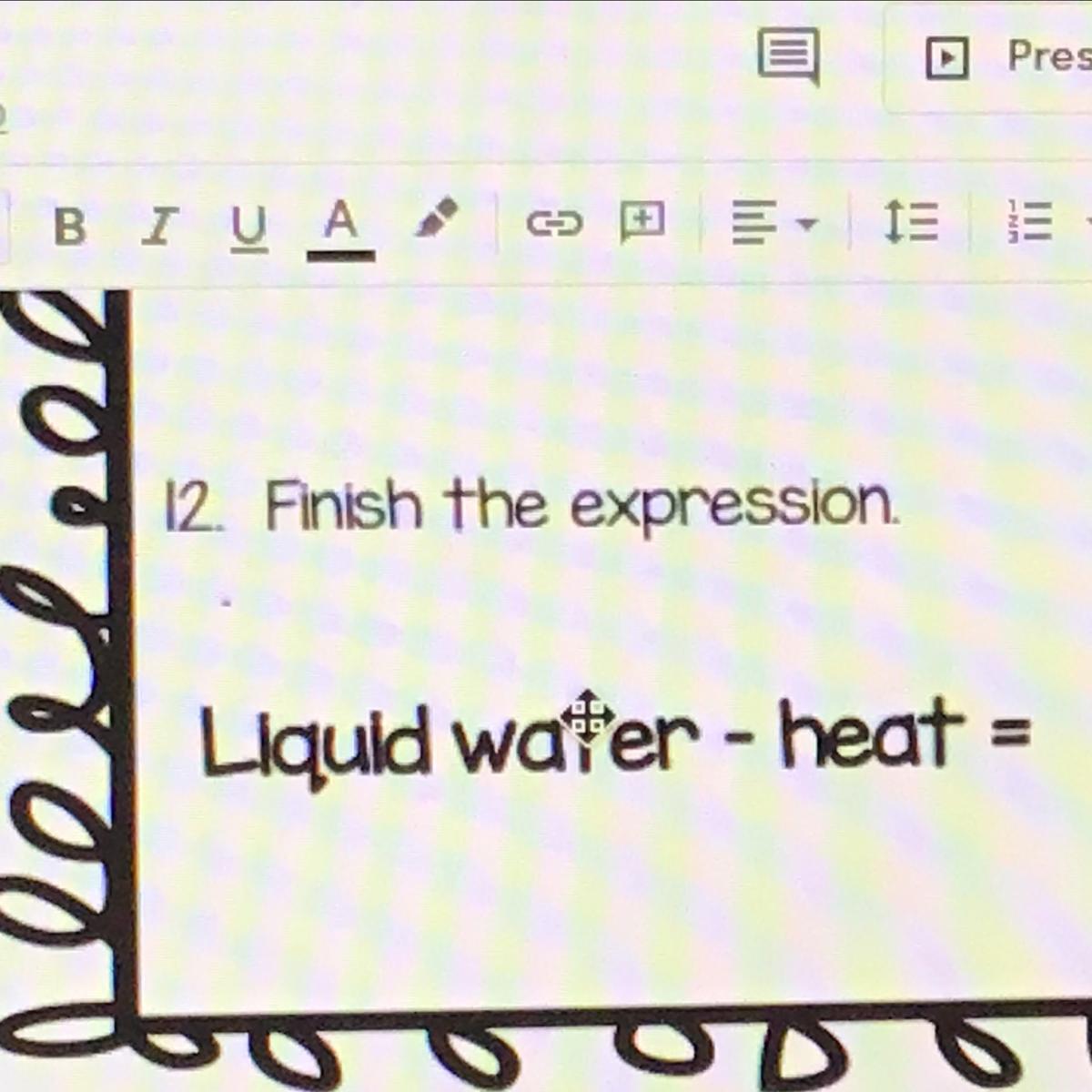
Answers
Answer:
cold or ice?
Explanation:
have a good day.
Calculate the pH of the solutions, given the following [H+], and then identify the solution as acidic, basic, or neutral.
[H+] = 1.2 x 10^-2 M
[H+] = 5.8 x 10^-9 M
[H+] = 3.92 x 10^-12 M
[H+] = 4.52 x 10^-5 M
Answers
pH = 1.92, acidic
pH = 8.24, basic
pH = 11.41, basic
pH = 4.34, acidic
To calculate the pH of a solution, we use the formula:
pH = -log[H+]
where [H+] is the concentration of hydrogen ions in moles per liter.
For [H+] = 1.2 x 10^-2 M:
pH = -log(1.2 x 10^-2) = 1.92
This solution is acidic.
For [H+] = 5.8 x 10^-9 M:
pH = -log(5.8 x 10^-9) = 8.24
This solution is basic.
For [H+] = 3.92 x 10^-12 M:
pH = -log(3.92 x 10^-12) = 11.41
This solution is basic.
For [H+] = 4.52 x 10^-5 M:
pH = -log(4.52 x 10^-5) = 4.34
This solution is acidic.
So, the pH and nature of the solutions are:
pH = 1.92, acidic
pH = 8.24, basic
pH = 11.41, basic
pH = 4.34, acidic
For more such questions on pH
https://brainly.com/question/17218129
#SPJ11
