Answers
A.1
B.4
C.5
D.3
E.2
Related Questions
9.25 mol C2H6 reacts with 15.0 mol 02 according to the equation below:
262H6 + 702 —> 4C02 + 6H20
What mass of carbon dioxide forms during the reaction of 9.25 moles C2H6?
[?] g CO2

Answers
Answer:
Mass= 351.5kg
Explanation:
From the question
mole = 9.25mol.
Molar mass = C2H6 = 16×2+1×6=32+6=38molg.
Mass = ?
\(n = \frac{mass}{moler \: mass} \)
\(9.25 = \frac{m}{38} \\ = 9.25 \times 38 = m \\ = 351.5kg\)
therefore mass is 351.5kg
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
4) How many grams of carbon dioxide would be needed to produce 3.382 grams of
acetylene (C2H,) using the following reaction?
4 CO2(g) + 2 H20(g) → 2 C2H2(g) +5 02(g)
Answers
Answer:
11.4 g CO₂
Explanation:
Your chemical equation is:
4 CO₂ + 2 H₂O ⇒ 2 C₂H₂ + 5 O₂
You need to produce 3.382 g of acetylene. To find out how many grams of carbon dioxide you need, first convert grams of acetylene to moles using the molar mass. The molar mass of acetylene is 26.04 g/mol.
(3.382 g)/(26.04 g/mol) = 0.130 mol C₂H₂
Now, use the mole ratio between acetylene and carbon dioxide to convert from moles of acetylene to moles of carbon dioxide. You can find the mole ratio by looking at the chemical equation. The mole ratio is (4 mol CO₂)/(2 mol C₂H₂).
(0.130 mol C₂H₂) × (4 mol CO₂)/(2 mol C₂H₂) = 0.260 mol CO₂
Since you now have moles of carbon dioxide, you can convert to grams using the molar mass. The molar mass of carbon dioxide is 44.01 g/mol.
0.260 mol × 44.01 g/mol = 11.4 g CO₂
You will need 11.4 g of CO₂ to produce 3.382 g of C₂H₂.
The molecular weight of glucose is 180. 156 g/mol. If you wish to administer 205. 00 grams of glucose from a 0. 278 m glucose solution, what volume of solution will need to be dispensed?.
Answers
To administer 205.00 grams of glucose from a 0.278 m glucose solution, we would need to dispense 4.14 liters of the solution.
The following steps are required to calculate the answer:-
To calculate the volume of the glucose solution that needs to be dispensed, we can use the following formula:
mass of solute (g) = concentration (mol/L) x volume (L) x molecular weight (g/mol)
We are given the mass of the solute (glucose) as 205.00 grams, and the molecular weight of glucose is 180.156 g/mol. We also know the concentration of the glucose solution, which is 0.278 m (mol/L).
Let's rearrange the formula to solve for volume:
volume (L) = mass of solute (g) / (concentration (mol/L) x molecular weight (g/mol))
Substituting the values we know, we get:
volume (L) = 205.00 g / (0.278 mol/L x 180.156 g/mol)
Simplifying the expression:
volume (L) = 4.14 L
Therefore, to administer 205.00 grams of glucose from a 0.278 m glucose solution, we would need to dispense 4.14 liters of the solution.
To know more about Molecule visit :-
https://brainly.com/question/475709
#SPJ1
What is the IUPAC name of Brady's reagent, Tollen reagent and Fehlings solution
Answers
Answer:
IUPAC name literally is a chemical organic name given to an organic compound bashing on the IUPAC naming rules.
• Brady's reagent → 2,4-dinitrophenylhydrazine
It is used to confirm presence of carbonyl compounds (compounds with a carbonyl carbon).
• Tollen's reagent → Silver ammonical nitrate solution
It is used to distinguish between terminal and non-terminal alkyns (compounds with carbon to carbon tripple bonds)
• Fehling's solution → Copper (II) sulphate and Silver nitrate solution [ Copper (I) nitrate solution ]
It is used to distinguish between alkanols and aldehydes.
Also it distinguishes between terminal and non terminal alkyns
\({}\)
Bas +
PtF2 →
BaF2 +
Pts
Need to balance it

Answers
it is already balanced
REACTANTS
Barium sulfide (BaS) + platinum (Ii) fluoride
PRODUCT
Barium fluoride (BaF2) + Cooperite (PtS)
Hope this answer helps you dear! take care
Please help
1. The atomic number tells us how many ____________ an element has.
protons
neutrons
electrons
Answers
atomic number tells us how many neutrons an element has
The density of ethanol, C2H5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produce 18.2 g of CO2 according to the following chemical equation?
C2H5OH(l) + 3 O2(g) → 2 CO2(g) + 3 H2O(l)
Answers
Answer: 12.1 ml of ethanol is needed
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}\)
\(\text{Moles of} CO_2=\frac{18.2g}{44g/mol}=0.414moles\)
\(C_2H_5OH(l)+3O_2(g)\rightarrow 2CO_2(g)+3H_2O(l)\)
According to stoichiometry :
2 moles of \(CO_2\) is produced by = 1 mole of \(C_2H_5OH\)
Thus 0.414 moles of \(CO_2\) is produced by=\(\frac{1}{2}\times 0.414=0.207moles\) of \(C_2H_5OH\)
Mass of \(C_2H_5OH=moles\times {\text {Molar mass}}=0.207moles\times 46.07g/mol=9.54g\)
Volume of ethanol = \(\frac{\text {Mass of ethanol}}{\text {density of ethanol}}=\frac{9.54g}{0.789g/ml}=12.1ml\)
12.1 ml of ethanol is needed to produce 18.2 g of \(CO_2\)
I need help with this homework thank you

Answers
What is the relationship between pressure and temperature?
PLEASEEE HELPPP!!!!!
Answers
Answer:
i forgot
Explanation:
Answer:The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law). The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law).
Explanation:
brainliest?
How do ionic bonds differ from covalent bonds?
Answers
Given the following equation: Mg + 2HCI → MgCl₂ + H₂
How many moles of H₂ can be produced by reacting 2 moles
of HCI?
Answers
Taking into account the reaction stoichiometry, 1 mole of H₂ can be produced by reacting 2 moles of HCI.
Reaction stoichiometryIn first place, the balanced reaction is:
Mg + 2 HCl → MgCl₂ + H₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Mg: 1 moleHCl: 2 molesMgCl₂: 1 moleH₂: 1 moleMoles of H₂ producedBy reaction stoichiometry 2 moles of HCl form 1 mole of H₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Which element has a higher ionization energy than silicon? Magnesium, Germanium, Sodium, or Phosphorus
Answers
Answer:
Phosphorus
Explanation:
Phosphorus will have higher ionization energy than silicon from the given choices.
Ionization energy is the energy required to remove the most loosely held electron in an atom.
Based on the periodic trends:
Across the period, ionization energy increases from left to rightDown a group, ionization energy decreases.Since phosphorus is the element in the right most part after silicon, it has higher ionization energy
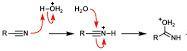
Acid-catalyzed hydrolysis of a nitrile to give a carboxylic acid occurs by initial protonation of the nitrogen atom, followed by nucleophilic addition of water. Draw curved arrows to show the movement of electrons in this step of the reaction mechanism.
Answers
Answer:
See explanation and image attached
Explanation:
The acid-catalyzed hydrolysis of a nitrile to give a carboxylic acid occurs by initial protonation of the nitrogen atom. This step is shown in the image attached.
The next step is the nucleophilic addition of water. The task is to show the movement of electrons in this step of the reaction mechanism. This was clearly shown in the image attached to this answer.
1. A 55.0g sample of iron(II) filings reacts with 23.8g of powdered sulfur (Sa). What is
the limiting reagent, excess reagent, and theoretical yield?
Answers
Due to its smaller quantity relative to iron(II) filings, sulphur acts as the limiting reagent in the reaction between iron(II) filings and iron. Since sulphur and iron(II) filings have a mole ratio of 1.74:1, we can determine the theoretical yield.
We calculate the amount of sulphur present in 0.742 mol by dividing the sulfur's mass (23.8 g) by its molar mass (32.06 g/mol). This is multiplied by the mole ratio, which results in a theoretical yield of 1.33 mol.
By dividing the moles of iron(II) filings (0.983 mol) by their molar mass (55.85 g/mol), one may calculate the theoretical yield of iron(II) filings, which is 54.6 g. Calculated as the difference between the original amount and the predicted yield (55.0 g - 54.6 g), the surplus iron(II) filings total 0.4 g.
Learn more about reagent at:
https://brainly.com/question/28463799
#SPJ1
How much kinetic energy does a 6.08 kg ball have if it's moving with a speed of 1.14 m/s?
Answers
Answer: KE = 8.6917248 J
I'm not for sure but I am pretty positive this is the answer
The balanced equation for the decomposition of dinitrogen pentoxide (N₂Os) to nitrogen dioxide (NO₂) and oxygen
(0₂) is shown.
The mass of one mole of each compound is given in the table.
Compound Molar Mass (grams)
N₂O5
108
NO₂
0₂
46
2 N₂O5-4 NO₂+0₂
32
Based on the balanced equation, use the menus to complete the statement to demonstrate the conservation of
mass.
When 216 grams of N₂Os completely decomposes, 184 grams
will be produced.
of NO₂ and 32 grams
of 0₂

Answers
Based on the law of conservation of mass, when 216 grams of N₂O5 completely decomposes, 184 grams of NO₂ will be produced and 32 grams of O₂.
What is the mass of the products that will be obtained?The mass of the products that will be obtained is calculated as follows:
The number of moles of N₂O5 in 216 grams:
216 g N₂O5 x (1 mol N₂O5/108 g N₂O5) = 2 moles N₂O5
From the balanced equation, 2 moles of N₂O5 will produce 4 moles of NO₂.
Therefore, the mass of NO₂ produced is as follows:
4 moles NO₂ x 46 g NO₂/1 mol NO₂ = 184 g NO₂
Based on the law of conservation of mass, when 216 grams of N₂O5 decomposes, 184 grams of NO₂ will be produced, and the remaining mass will be oxygen:
216 g N₂O5 - 184 g NO₂ = 32 g O₂
Learn more about mass of product at: https://brainly.com/question/24680018
#SPJ1
28.25 What two aldoses yield D-xylose on Wohl degradation?
Answers
56.5
Explanation:
the degradation would be 28.25 times 2
I have a balloon that has a volume of 0.5 L at a pressure of 0.5 atm. What is the new volume at a pressure of 1 atm?
I have a container at a volume of 2 L and at a temperature of 125 C. What is the new temperature of the container at a volume of 2 L?
A sample of helium gas in a balloon is compressed from 4.0 L to 2.5 L at a constant temperature. If the initial pressure was 3.0 atm at 4.0 L, what is the new pressure at 2.5 L?
A container has 50 mL of nitrogen at 25 C. What will be the volume if the new temperature if 60 C?
Answers
1)The new volume at a pressure of 1 atm is 0.25 L.
2)The new temperature of the container at a volume of 2 L is approximately 398°C.
3)The new pressure at 2.5 L is approximately 4.8 atm.
4)The new volume at a temperature of 60°C is approximately 55.8
1)To solve these gas law problems, we can use the ideal gas law equation, which states:
PV = nRT,
where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature in Kelvin.
Balloon volume at a pressure of 0.5 atm:\(V_1\) = 0.5 L, \(P_1\)= 0.5 atm.
New volume at a pressure of 1 atm:\(P_2\) = 1 atm.
We can use the relationship\(P_1V_1 = P_2V_2\) to find the new volume (\(V_2\)).
(0.5 atm)(0.5 L) = (1 atm)(\(V_2\))
\(V_2\) = 0.25 L.
Therefore, the new volume at a pressure of 1 atm is 0.25 L.
2)Container volume: \(V_1\) = 2 L, \(T_1\)= 125°C.
New temperature at the same volume: \(V_2\) = 2 L.
We can use the relationship\(V_1\)/\(T_1\) = \(V_2\)/\(T_2\) to find the new temperature (\(T_2\)).
(2 L)/(125 + 273) K = (2 L)/(\(T_2\) + 273) K
Solving for\(T_2\), we get \(T_2\) ≈ 398°C.
Therefore, the new temperature of the container at a volume of 2 L is approximately 398°C.
3)Initial volume: \(V_1\)= 4.0 L, \(P_1\) = 3.0 atm.
Final volume: \(V_2\) = 2.5 L.
Since the temperature (T) is constant, we can use the relationship \(P_1\)\(V_1\) = \(P_2V_2\) to find the new pressure (\(P_2\)).
(3.0 atm)(4.0 L) = (\(P_2\))(2.5 L)
\(P_2\) ≈ 4.8 atm.
Therefore, the new pressure at 2.5 L is approximately 4.8 atm.
4)Initial volume: \(V_1\)= 50 mL, \(T_1\) = 25°C.
New temperature: \(T_2\) = 60°C.
We need to convert the temperatures to Kelvin.
\(T_1\)= 25 + 273 = 298 K, \(T_2\) = 60 + 273 = 333 K.
We can use the relationship \(V_1/T_1 = V_2/T_2\) to find the new volume (\(V_2\)).
(50 mL)/(298 K) = (\(V_2\))/(333 K)
\(V_2\) ≈ 55.8 mL.
Therefore, the new volume at a temperature of 60°C is approximately 55.8
Know more about volume here:
https://brainly.com/question/27710307
#SPJ8
Gallium (Ga, 69.723 g/mol) is a metalloid obtained from its salts during the smelting of ores of other elements, like Zinc. has broad applicability in the electronics industry. It is also used as a safe replacement for mercury in thermometers as it melts at 29.8 °C and has a heat of fusion of 5.59 kJ/mol. What is the entropy change of 22 g of gallium in J/K as it melts when placed on a surface at 29.8°C?
Answers
Answer:
4.255 J/ K
Explanation:
Given data :
mass of Gallium = 16 gm
molar mass = 69.723 g/mol
hence no of moles = 16 / 69.723 = 0.23 moles --------- ( 1 )
Δh ( heat fusion ) = 5.59 KJ/mol
Temperature = 29.8°C = 302.8 k
Determine the entropy change of gallium
Δs ( entropy change ) = heat fusion / temperature
= ( 5.59 * 1000) J/mol / 302.8 = 18.46 J/k*mol
Hence entropy change of gallium
= Δs * no of Gallium moles
= 18.46 * 0.23 = 4.255 J/ k
I hope this is right haha
what is the empirical formula for Barium chloride dihydrate
Answers
what is the empirical formula for Barium chloride dihydrate
answer
BaCl2.2H2O
Copper crystallizes in a face-centered cubic unit cell. The density of copper is 8.94 g/cm3. Calculate the length of the edge of the unit cell in pm.
a) 461 pm
b) 361 pm
c) 261 pm
d) 161 pm
e) None of the above
Answers
Answer:
361.4 pm is the length of the edge of the unit cell
Explanation:
First, let's calculate the average volume each atom is taking. Start with calculating how many moles of copper we have in a cubic centimeter by looking up the atomic weight. Atomic weight copper = 63.546 Now divide the mass by the atomic weight, getting 8.94 g / 63.546 g/mol = 0.140685488 mol And multiply by Avogadro's number to get the number of atoms: 0.140685488 * 6.022140857x10^23 = 8.472278233x10^22 Now examine the face-centered cubic unit cell to see how many atoms worth of space it consumes. There is 1 atom at each of the 8 corners and each of those atoms is shared between 8 unit cells for for a space consumption of 8/8 = 1 atom. And there are 6 faces, each with an atom in the center, each of which is shared between 2 unit cells for a space consumption of 6/2 = 3 atoms. So each unit cell consumes as much space as 4 atoms. Let's divide the number of atoms in that cubic centimeter by 4 to determine the number of unit cells in that volume. 8.472278233x10^22 / 4 = 2.118069558x10^22 Now calculate the volume each unit cell occupies. 1 cm^3 / 2.118069558x10^22 = 4.721280262x10^-23 cm^3 Let's get the cube root to get the length of an edge. (4.721280262x10^-23 cm^3)^(1/3) = 3.61426x10^-08 cm Now let's convert from cm to pm. 3.61426x10^-08 cm / 100 cm/m * 1x10^12 pm/m = 361.4 pm Doing an independent search for the Crystallographic Features of Copper, I see that the Lattice Parameter for copper at at 293 K is 3.6147 x 10^-10 m which is in very close agreement with the calculated amount above. And since metals expand and contract with heat and cold, I assume the slight difference in values is due to the density figure given being determined at a temperature lower than 293 K.
3. The best instrument used to magnify small objects up to 20 times is a
A. magnifying lens
B. compound microscope
C. electron microscope
D. dissecting scope
Answers
Answer:
Explanation:
a piano white
The best instrument used to magnify small objects up to 20 times is a compound microscope. Therefore, option B is correct.
What is compound microscope ?The optical microscope, also known as a light microscope, creates enlarged pictures of tiny objects by using visible light and a set of lenses.
Viewing tiny samples that are invisible to the human eye is done with compound microscopes. Normally, a slide with these samples on it is used in a microscope. When utilizing a stereo microscope, bigger items like rocks or flowers may fit beneath the microscope with greater room, and slides are not necessary.
Because it has two different types of lenses that work together to magnify an item, the conventional light microscope used in laboratories is referred to as a compound microscope.
Thus, option B is correct.
To learn more about compound microscope, follow the link;
https://brainly.com/question/1560613
#SPJ2
Explain how temperature, concentration and a
catalyst will affect the rate of a reaction.
What are the three points of collision theory
that are required for a reaction to happen?

Answers
Answer:
Temperature, concentration, and a catalyst can all affect the rate of a chemical reaction.
Temperature: Increasing the temperature generally increases the rate of a reaction. This is because higher temperatures provide more kinetic energy to the reactant particles, causing them to move faster and collide more frequently. With increased collision frequency, the chances of successful collisions with sufficient energy to overcome the activation energy barrier and proceed with the reaction are also increased. As a result, the reaction rate typically increases with temperature.
Concentration: Increasing the concentration of reactants generally increases the rate of a reaction. When the concentration of reactant particles is higher, they become more crowded, increasing the likelihood of collisions between reactant particles. With more collisions occurring, there is a higher probability of successful collisions leading to a reaction. Therefore, higher reactant concentrations generally result in a higher reaction rate.
Catalyst: A catalyst is a substance that increases the rate of a reaction by providing an alternative reaction pathway with a lower activation energy. Catalysts themselves are not consumed during the reaction and do not undergo any permanent changes. They work by providing an alternative route that requires less energy for the reactants to reach the transition state. This lowers the activation energy barrier, making it easier for the reaction to occur. By providing an alternative pathway, catalysts increase the rate of reaction without being consumed in the process.
Regarding collision theory, the three key points required for a reaction to happen are:
Collision: Reactant particles must collide with each other for a reaction to occur. Collisions bring the reactant particles in close proximity, allowing them to interact and potentially form new chemical bonds.
Energy: Colliding particles must possess enough energy, equal to or greater than the activation energy, for the reaction to take place. Activation energy is the minimum energy required for the reactant particles to break existing bonds and initiate the formation of new bonds. Only collisions with sufficient energy can overcome the activation energy barrier and lead to a reaction.
Orientation: In addition to sufficient energy, the collision between reactant particles must occur with the correct orientation. This means that the particles must collide in a way that allows the necessary atoms or groups to come into contact and form new bonds. If the collision occurs with an incorrect orientation, the particles may simply bounce off each other without any reaction taking place.
In summary, according to collision theory, for a reaction to happen, reactant particles must collide with sufficient energy and the correct orientation. Temperature and concentration affect the rate of reaction by influencing collision frequency, while a catalyst provides an alternative reaction pathway with lower activation energy.
The density of a gas sample is 1.29 g/L. How many grams of gas are there in 255 L
Answers
What is the mass of 1.0 × 10^9 molecules of aspartame?
Answers
Answer:
294.3 g/mol b.
The mass of 1.0*10^9 molecules of aspartame is 2.943*10^11 g/mol
ASPARTAME
Aspartame is used as an artificial non-saccharide sweetener 200 times sweeter than sucrose, and is commonly used as a sugar substitute in foods and beverages.
CALCULATION
mass of one molecule of aspartame = 294.3g/mol
mass of 1.0*10^9 molecules of aspartame =294.3*(1.0*10^9) g/mol
=2.943*10^11 g/mol
refer https://brainly.com/question/25225559
#SPJ2
What is the maximum number of electrons that can have the following set of quantum numbers?
n = 4, l = 2, and ms = +½
Answers
Answer:
5
Explanation:
First, list out the 4 quantum number symbols, which are:
n, l, ml, ms
since the n value is 4 and the l value is 2, the orbital name would be 4d. a 4d orbital can normally hold 10 electrons, but ms must be +1/2. since there are only 5 electrons with +1/2 spin (the other 5 having -1/2 spin), only 5 electrons can have those quantum numbers
What sample at STP has the same number of molecules as 5 L of NO2
Answers
Answer:
5l NO
2
at STP
No. of molecules=
22.4
5
mol=
22.4
5
×N
A
molecules
A) 5ℊ of H
2
(g)
No. of moles=
2
5
mol=
2
5
×N
A
molecules
B) 5l of CH
4
(g)
No. of moles of CH
4
=
22.4
5
mol=
22.4
5
N
A
molecules
C) 5 mol of O
2
=5N
A
O
2
molecules
D) 5×10
23
molecules of CO
2
(g)
Molecules of 5l NO
2
(g) at STP=5l of CH
4
(g) molecules at STP
Therefore, option B is correct.
Was this answer helpful?
How to I make a strength potion?
Answers
Answer:
Hopefully this helps!
Explanation:
In the Brewing Stand menu, you place ingredients in the top box and the potions are created in the bottom three boxes. To make a Potion of Strength (3:00), you will need 1 water bottle, 1 nether wart, and 1 blaze powder.
a.
Neutrons have a neutral charge, a mass of 1 amu, and are found in an atom's ______
b.
Protons are a little smaller than neutrons, they still have a mass of 1 amu though. They are found inside an atom's nucleus and carry a
_______ charge.
C.
Electrons almost have no mass, it's so small we say it is 0 amu. They sail around the nucleus in the _______ They have a ______
charge.
Answers
Neutrons have a neutral charge, a mass of 1 amu, and are found in an atom's Nucleus , Protons are a little smaller than neutrons, they still have a mass of 1 amu though. They are found inside an atom's nucleus and carry a positive charge, Electrons almost have no mass, it's so small we say it is 0 amu. They sail around the nucleus in the orbit, They have a negative charge
Atoms of all elements except for most atoms of hydrogen have neutrons in their nucleus unlike protons and electrons, which are electrically charged, neutrons have no charge they are electrically neutral. that's why the neutrons in the diagram above are labeled n⁰ and the zero stand for zero charge neutron has a neutral charge
Protons are found in the nucleus of the atom and this is a tiny, dense region at the center of the atom protons have a positive electrical charge of one (+1) and a mass of 1 atomic mass unit proton carry positive charge in the nucleus of atom
Electrons are one of three main types of particles that make up atoms and protons and neutrons, which consist of smaller, simpler particles, electrons are fundamental particles that do not consist of smaller particles electron has 0 amu and they have negative charge around the nucleus in the in the orbit called electron
Know more about Neutrons, Protons, Electrons
https://brainly.com/question/28667559
#SPJ1
