help asap!!! Compare and contrast synthesis and decomposition reactions.
this will be copied and pasted FYI If answered already comment, please
Answers
Answer:
synthesis and decomposition has a high scientific value as synthesis and decomposition are one of the most important types of chemical reactions to occur in the nature. A chemical reaction is defined as the formation or breaking of chemical bonds between atoms to form new combinations of atoms. The atoms or combination of atoms involved in a chemical reaction is referred to as reactants and the newly formed substances are known as products. All the chemical reactions occur in the biological system are categorized into four types; synthesis reactions, decomposition reactions, exchange reactions, and reversible reactions. In this article, the difference between synthesis and decomposition will be discussed broadly.
Explanation:
What are the differences between Synthesis and Decomposition?•
Decomposition is the opposite of synthesis.•
Synthesis is the process of the formation of new bonds between reactants to form new products, whereas decomposition is the breaking of chemical bonds within reactants to form different products.•
Synthesis requires energy, whereas decomposition release energy.•
Decomposition reactions are collectively called catabolism, whereas synthesis reactions are called anabolism.•
Synthesis involved in the growth of body parts and repair body tissues. Decomposition takes place during the digestion of foods.
Related Questions
how many moles of helium atoms are there in 0.02g of helium
Answers
The number of moles of helium atoms there in 0.02g of helium is 0.00499 mol
What are moles?
"The mole is a SI unit of measurement that is used to calculate the quantity of any substance".
"Molar mass of many compounds can be calculated by dividing the mass of the compound by the number of moles of the compound".
Mass is a physical body's total amount of matter. Mass is defined as the sum of the moles of the material and the compound's molar mass.
Moles can be calculated by dividing the mass with the molar mass
Given the mass is 0.02 grams
The molar mass of helium is 4.002602
Molar mass is calculated by seeing the molecular weight and number of moles of that atoms.
Moles = mass / molar mass
0.02 / 4.002 = 0.00499 mol
Thus, the number of moles in helium are 0.00499 mol.
To learn more about moles, refer to the link:
https://brainly.com/question/21323029
#SPJ2
Calculate ΔH for the reaction CO(g) + H2(g) + O2(g) → CO2(g) + H2O(g)
Given:
2 C(s) + O2(g) → 2 CO(g)... ∆H = -222 kJ
C(s) + O2(g) → CO2(g)... ∆H = -394 kJ
2 H2(g) + O2(g) → 2H2O(g)... ∆H = -484 kJ
Answers
The value of ΔH for the reaction CO(g) + H₂(g) + O₂(g) → CO₂(g) + H₂O(g) is -1272 kJ.
To calculate the enthalpy change (ΔH) for the given reaction, we can use Hess's law, which states that the overall enthalpy change of a reaction is equal to the sum of the enthalpy changes of the individual reactions involved.
Given the enthalpy changes:
1. 2 C(s) + O₂(g) → 2 CO(g)... ∆H = -222 kJ
2. C(s) + O₂(g) → CO₂(g)... ∆H = -394 kJ
3. 2 H₂(g) + O₂(g) → 2 H₂O(g)... ∆H = -484 kJ
We need to manipulate these reactions to obtain the desired reaction:
1. Reverse reaction 2: CO₂(g) → C(s) + O₂(g)... ∆H = +394 kJ
2. Multiply reaction 2 by 2 to balance carbon atoms: 2 CO₂(g) → 2 C(s) + 2 O₂(g)... ∆H = -788 kJ
3. Leave reaction 3 unchanged: 2 H₂(g) + O₂(g) → 2 H₂O(g)... ∆H = -484 kJ
By adding reactions 2 and 3, we obtain the desired reaction:
CO(g) + H₂(g) + O₂(g) → CO₂(g) + H₂O(g)... ∆H = -788 kJ + (-484 kJ) = -1272 kJ
Therefore, the value of ΔH for the given reaction is -1272 kJ.
Learn more about Reaction
brainly.com/question/14025220
#SPJ11
Which pair must represent atoms of the same element?

Answers
Answer nuber 3
Explanation:
What do these two changes have in common? a piece of pear turning brown and bleaching clothes
Both are caused by cooling.
Both are changes of state.
Both are chemical changes.
Both are caused by heating.
Answers
Answer:
Neither of these changes are caused by cooling, but both are chemical changes.
Select the best choice from the drop-down menus. A gas mixture with a total pressure of 5 atm contains 1.39 atm of nitrogen gas, 2.5 atm of helium gas, and some carbon dioxide gas. The table below shows the set of values given for the carbon dioxide gas: Volume 7.10 L Temperature 304 K The partial pressure of carbon dioxide gas is Select... ✓ atm. Select... The number of moles of the carbon dioxide gas is Select...
Answers
Answer: a. 1.11 atm, 0.43 mol
Explanation:
What is the answer of the ice cream activity of integration
Answers
The ice cream activity of integration is that it demonstrates how integration can be used to find the area under a curve or the total quantity of a certain variable, such as the amount of ice cream consumed.
This activity involves plotting the ice cream consumption over time on a graph, with the x-axis representing time and the y-axis representing the amount of ice cream consumed. The curve formed by the data points represents the rate of ice cream consumption.
The goal of this activity is to find the total amount of ice cream consumed during a specific time interval. To do this, you can use integration, which is a mathematical technique for finding the area under a curve.
By integrating the function that describes the curve, you can determine the total ice cream consumed during the given time period. This activity helps to illustrate the concept and application of integration in real-life situations.
To know more about integration click on below link:
https://brainly.com/question/30900582#
#SPJ11
Question 2 of 30
A television commercial shows happy people while describing some medical
symptoms. These symptoms include feeling tired and sad. The medication
being advertised by the commercial was approved by the FDA to treat a
disease that causes these symptoms. The narrator says that it is available by
prescription only and contains 1% of the active ingredient. What can you infer
about this medication?
OA. The people in the commercial are happy because they were
treated by the medication.
B. The medication would be more effective if it contained 10% of the
active ingredient.
C. Anyone who has the symptoms should request a prescription
from his or her doctor.
D. The medication can treat people who have the disease described.
Answers
The medication can treat people who have the disease described. According to the commercial, the drug has FDA approval to treat a condition whose symptoms are listed.
Additionally, the narrator notes that the medication only comes with a prescription and has 1% of the active substance. We can deduce from this information that the drug can effectively treat persons who have the condition generating these symptoms, but obtaining it requires a prescription. The commercial provides no support for the other possibilities.
Therefore, the correct option is D.
Learn more about Medication, here:
https://brainly.com/question/11098559
#SPJ1
The electron configuration of an element is 1s22s22p63s1. Describe what most likely happens when an atom of this element comes near an atom having seven valence electrons.
PLEASE HELP!!!!!!!
Answers
Answer:
The element with electron configuration 1s² 2s² 2p⁶ will most likely not react with an element having seven Valence electrons.
The electron configuration of the element in discuss is 1s² 2s² 2p⁶.
The element has enough electrons to fill it's energy level, n = 2 shell.
In essence, the element in discuss is unreactive as it has attained it's octet configuration and as such is neither in need of an electron nor ready to donate an electron.
As such, although an atom having seven Valence electrons is highly electronegative and as such is an electron attractor, the element with the full octet configuration does not react with it.
This unreactive nature of noble gases is attributed to the full octet configuration of noble gases.
P.S: The electron configuration above is the electron configuration of Neon, Ne.
How many moles of argon are there in a 22.4 L sample of gas at 101.3 kPa and 0 C?
Show your work
Answers
Answer:
0.999 moles of argon (3 s.f.)
Explanation:
To determine the number of moles of argon in a gas sample, we can use the ideal gas law equation:
Ideal Gas Law\(\boxed{PV=nRT}\)
where:
P is the pressure measured in kilopascals (kPa).V is the volume measured in liters (L).n is the number of moles.R is the ideal gas constant (8.31446261815324 kPa L mol⁻¹ K⁻¹).T is the temperature measured in kelvin (K).Since we are finding "n", rearrange the equation for n:
\(\implies n=\dfrac{PV}{RT}\)
As the temperature has to be measured in kelvin, convert the temperature from Celsius to kelvin by adding 273.15:
\(\implies \sf 0^{\circ}=0+273.15\;K=273.15\;K\)
Therefore, the values to substitute into the equation are:
P = 101.3 kPaV = 22.4 LR = 8.31446261815324 L kPa mol⁻¹ K⁻¹T = 273.15 KSubstitute the values into the formula and solve for n:
\(\implies n=\sf \dfrac{101.3\;kPa \cdot 22.4\;L}{8.314 462...L\;kPa\;mol^{-1}\;K^{-1} \cdot 273.15\;K}\)
\(\implies n=\sf \dfrac{101.3\cdot 22.4}{8.314 462...\;mol^{-1}\cdot 273.15}\)
\(\implies n=\sf \dfrac{2269.12\;mol}{2271.09546...}\)
\(\implies n=\sf 0.99913017...\;mol\)
\(\implies n=\sf 0.999\;mol\;(3\;s.f.)\)
Therefore, there are 0.999 moles of argon (to three significant figures) in a 22.4 L sample of gas at 101.3 kPa and 0°C.
Which question would most likely be studied by a physicist?
A. Were there ever any living organisms on Mars?
B. How can the forces on a space probe be controlled so it will land
on Mars?
O C. What type of substances make up the soil on Mars?
O D. Should the government spend taxpayers' money to send space
probes to Mars?
Answers
Answer:A
Explanation: Were there any living organisms is the answer because physics is the study of matter,its motion and behaviour of space and time and some other topics like energy and force.
Answer:
B
Explanation:
Forces and vectors on a probe are a physics thing
a claim about arrangements of electrons and properties within a family elements
Answers
A claim about the arrangements of electrons and properties within a family of elements is described below:
elements in the same family have the same number of outermost shell electronselements in the same family have similar chemical properties due to them having the same arrangements of electronsWhat are families of elements?Families of elements refer to elements that are found in the same group in the periodic table.
Elements that belong to the same family have the same arrangement of electrons.
The families of elements are found in the vertical columns knowns as groups. They have the same physical properties because they have the same number of e; electrons in their outermost shell.
For example, elements belonging to group 1 have one valence electron and show similarity in their chemical properties.
Learn more about families or groups of elements at: https://brainly.com/question/13870873
#SPJ1
what is the mass of carbon dioxide which contain the same number of molecules as are contained in 14 gram of oxygen?
Answers
Answer:
Mass of CO2 WILL BE ~ 9.33 g
Explanation:
Moles of O2 = 14/18
Let the mass of CO2 be x
Then moles of CO2 will be = x/12
moles of CO2 = moles of O2
x/12 = 14/16
x = 9.33 grams
After the change was made at time t1, the partial pressure of SO3 increased while the pressure of )2 decreased. Explain
Answers
Based on the information provided, it seems that a chemical reaction occurred at time t1 that involved the gases SO3 and O2. The change in partial pressure of these gases indicates that a shift occurred in the equilibrium of the reaction.
One possible explanation for the increase in partial pressure of SO3 is that the reaction was exothermic and favored the production of SO3 at the temperature and pressure conditions present. As the reaction proceeded, more SO3 was formed and the partial pressure increased. At the same time, the decrease in partial pressure of O2 suggests that it was consumed in the reaction to form SO3. This decrease could occur if the reaction was consuming O2 faster than it was being supplied, or if the equilibrium of the reaction favored the consumption of O2 over the production of SO3.
Overall, the change in partial pressures indicates that the chemical reaction caused a shift in the equilibrium of the system towards the production of SO3.
To know more about partial pressures, click here:-
https://brainly.com/question/31214700
#SPJ11
what is the ph of a solution that contains 11.7g of nacl for every 200 ml of solution?
Answers
Answer:
Explanation:
The pH of a solution that contains 11.7 g of NaCl for every 200 mL of solution cannot be determined from the information provided. The pH of a solution depends on the concentration of hydrogen ions (H+) present in the solution. The presence of NaCl in the solution does not directly affect the pH of the solution.
NaCl is a neutral salt, meaning it does not affect the pH of a solution when it is dissolved in water. In order to determine the pH of the solution, we would need to know the concentration of hydrogen ions (H+) in the solution.
Minerals are formed by which process? O magma cooling O fault lines moving O metamorphosis O sedimentation
Answers
A voltaic cell with a basic aqueous electrolyte is based on the oxidation of Cd(s) to Cd(OH)2(s) and the reduction of MnO4–(aq) to MnO2(s).
Answers
Answer:
See explanation
Explanation:
Oxidation half equation;
3Cd(s) + 6OH^-(aq) ------> 3Cd(OH)2(s) + 6e
Reduction half equation;
2MnO4^-(aq) + 8H^-(aq) + 6e --------> 2MnO2(s) + 4H2O(l)
Balanced reaction equation;
3Cd(s) + 6OH^-(aq) + 2MnO4^-(aq) + 8H^-(aq) ------> 3Cd(OH)2(s) + 2MnO2(s)
Number of electrons transferred = 6
How many moles is 3.01x1023 atoms of Nickle
Answers
Answer:
The answer is 0.5 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\ \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{3.01 \times {10}^{23} }{6.02 \times {10}^{23} } \\ \)
We have the final answer as
0.5 molesHope this helps you
How many liters in 9.87 moles of 0^3
Answers
The volume (in liters) in which 9.87 moles of ozone, O₃ can occupy is 221.09 liters
How do i determine the volume?From the question given above, the following data were obtained:
Number of mole of ozone, O₃ = 9.87 molesVolume of ozone, O₃ =?The volume of 9.87 moles of ozone, O₃ can be obtained as illustrated below:
From the ideal gas theory, we understood that:
1 mole of ozone, O₃ = 22.4 Liters
Therefore,
9.87 moles of ozone, O₃ = (9.87 moles × 22.4 Liters) / 1 mole
9.87 moles of ozone, O₃ = 221.09 liters
Thus, we can conclude that the volume is 221.09 liters
Learn more about volume:
https://brainly.com/question/225322
#SPJ1
4Al + 3O2 —> 2Al2 O3
10.7g of powdered Al is placed into a container containing 10.7g O2. What is the limiting reactant? How many grams of aluminum oxide can be produced? Calculate the mass of excess reactant that remains after the reaction is complete
Answers
Limiting reactant : Al
Al2O3 produced = 18.36 g
mass O2 remains = 11.904 g
Further explanationGiven
Reaction
4Al + 3O2 —> 2Al2O3
10.7 g O2
Required
Limiting reactant
mass Al2O3
Mass of excess reactant remains
Solution
mol Al : 10.7 : 27 = 0.396
mol O2 = 10.7 : 32 = 0.334
1. limiting reactant
mol : coefficient of Al : O2 = 0.396/4 : 0.334/3 = 0.099 : 0.111
Al as a limitng reactant(smaller ratio)
2. mass Al2O3
mol Al2O3 based on Al : 2/4 x 0.396 = 0.18
mass Al2O3(MW=102 g/mol) = 0.18 x 102 = 18.36 g
3. mass O2 remains
mol O2 reacted : 3/4 x 0.396 = 0.297
mol O2 remains = 0.669 - 0.297 =0.372
mass O2 remains = 0.372 x 32 = 11.904 g
Plz plz here
A student pours 25.0 mL of water into a graduated cylinder. She drops a plece of unknown metal with a mass of 18.9 g Into the cylinder. The
water level rises to 32.0 mL. The student uses the table to identify the metal
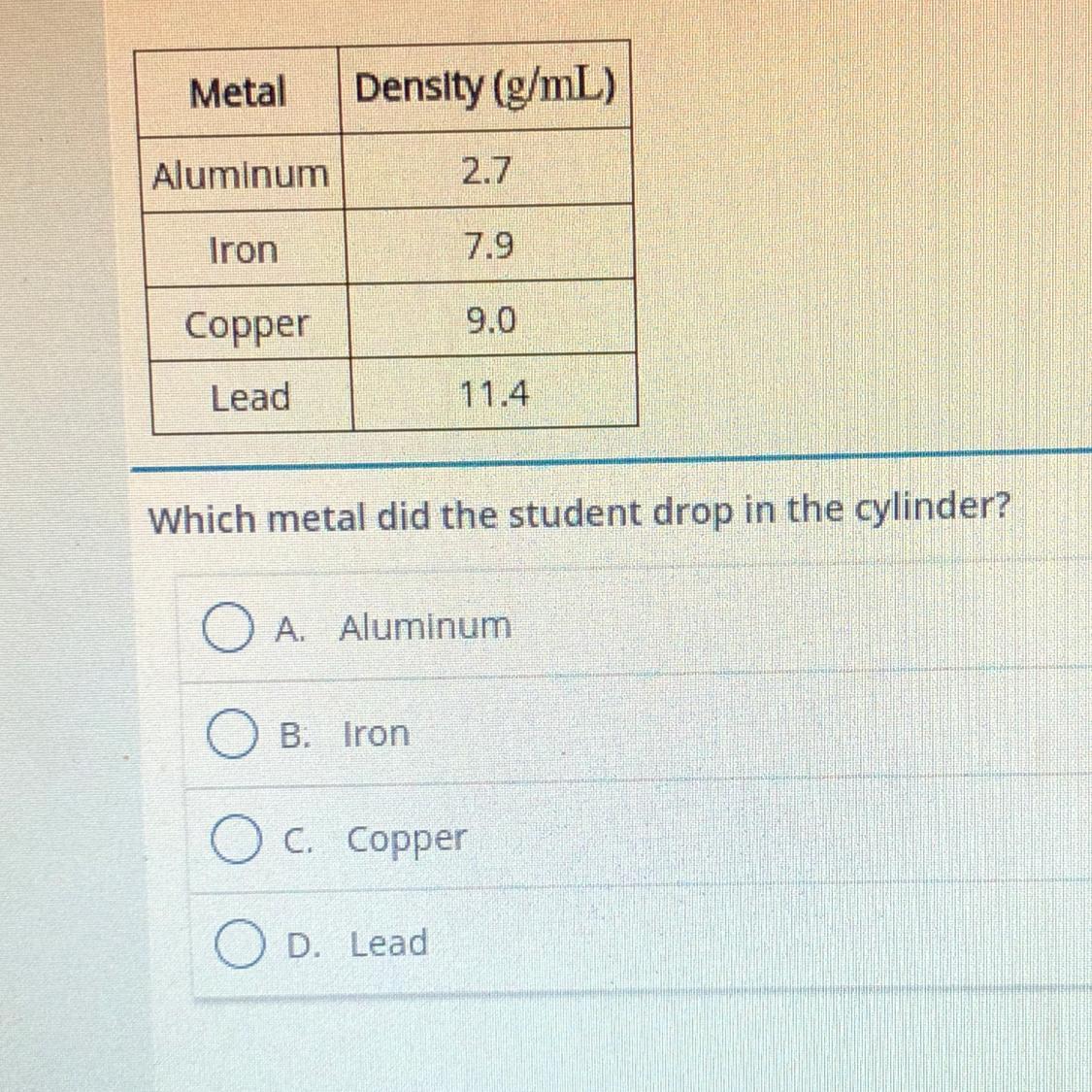
Answers
Answer:
Aluminum
Explanation
The volume of the metal is found by subtracting the final volume by the Intel volume giving 7
And the formula for density is mass divided by volume hence minus 7 by 18.9 giving 2.7
Hydrochloric acid has the
chemical formula HCI. Does this
information make you think that
hydrochloric acid is an element,
compound, or mixture
Answers
Answer:
its a mixture
Explanation:
I can't explain why
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
I need help asap!!! At least with the first part

Answers
Answer:
The correct answer -
a. Cd and Pb(NO3)2
b. Redox reactions
c. Pb and Cd(NO3)2
Explanation:
This is the reaction known as the redox or reduction-oxidation reaction of metals. In this particular reaction, there are two reactants Cadmium (III) in solid-state and lead (II) nitrate in the aqueous state. At the end of this reaction, the products that we get are lead (II) in solid-state and Cadmium (III) nitrate in the aqueous state.
cadmium (s)+ lead nitrate (aq) = lead (s) + cadmium nitrate (aq)
Cd (s) + Pb2+(aq) → Pb(s) + Cd2+(aq)
Here, Oxidizing agent is Pb2+ and the reducing agent is Cd.
You're paid $25 per hour for your job. How much would you earn in cents per second?
Answers
Answer:
0.694 cents per second
Explanation:
25x100=2500 cents per hour, 2500/60 = 41.67 per minute and 41.67/60=0.694 cents per second
In naming the compound PCl5, the prefix used with the second element is ____________________.
Answers
Answer:
\(\boxed {\boxed {\sf Hepta}}\)
Explanation:
We are given the formula:
\(PCl_5\)
This is a molecular formula, because it contains nonmetals.
1. Name the first element
The first element is phosphorous (P). Since this is the first element and there is only one, we don't need a prefix.
Phosphorous2. Second element
The second element is chlorine (Cl). It has a subscript of 5, so we must add the prefix of hepta-.
Phosphorous heptachlorineAdd the ending of -ide.
Phosphorous heptachlorideThe prefix used for the second element is hepta. The compound name is phosphorous heptachloride.
Groups of atoms with aligned magnetic poles are called.
Answers
Answer: Groups of atoms with aligned magnetic poles are called magnetic domains. Each domain contains an enormous number of atoms, yet the domains are too small to be seen with the unaided eye.
Explanation:
Oxygen Vacancies Enhancing Capacitive Properties of MnO2 Nanorods for Wearable Asymmetric Supercapacitors
Answers
By creating oxygen vacancies, we show how to increase the conductivity and capacitive performance of MnO2 intrinsically. The electrochemical performance of oxygen-deficient MnO2 nanorods (NRs) generated by a simple hydrogenation treatment is much better than that of the untreated MnO2 electrode.
what is an Asymmetric Supercapacitors?
The insufficient capability of energy-storage devices frequently stymies ongoing technical developments in different domains such as portable gadgets, transportation, and green energy. Asymmetric supercapacitors can extend their working voltage window beyond the thermodynamic breakdown voltage of electrolytes while solving the energy storage restrictions of symmetric supercapacitors by utilising two distinct electrode materials. This review provides in-depth expertise in this topic. To comprehend the wide-ranging research in this area, we first look at the basic energy-storage mechanisms and performance evaluation criteria for asymmetric supercapacitors.
learn more about Asymmetric Supercapacitors refer:
https://brainly.com/question/24466849
#SPJ4
6)
Which formula can represent
an
alkyne?
A)
C₃H6
B)
C₂H4
C)
C3H4
D)
C₂H6
Answers
Answer:
c)C3H4
Explanation:
Answer: C. C3H4
Explanation:
I got it wrong and castle learning showed me the answer
Describe what occurs when a metal is placed into an acid and the equation and what is occurring within it
Answers
When a metal is placed into an acid, a chemical reaction called a redox reaction occurs. In this process, the metal loses electrons (oxidation) and the acid gains electrons (reduction). This results in the formation of a salt and hydrogen gas is released. The general equation for this reaction is:
Metal + Acid → Salt + Hydrogen gas
For example, when zinc is placed into hydrochloric acid, the reaction is:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
In this reaction, zinc is oxidized to form zinc chloride (ZnCl₂) while hydrogen ions (H⁺) in the hydrochloric acid are reduced to form hydrogen gas (H₂).
The release of hydrogen gas can be observed as bubbles forming in the solution. This process demonstrates the reactivity of metals with acids, which depends on the metal's position in the reactivity series.
To know more about redox reaction refer here:
https://brainly.com/question/13293425#
#SPJ11
What mass, in grams, of H₂ can be produced from 35.0 g Na?
2 Na + 2 H₂O → 2 NaOH + H₂
6.15 g H₂
3.08 g H₂
1.54 g H₂
4.39 g H₂
Answers
molar mass of Na = 23.0 g/mol
moles of Na = mass/molar mass = 35.0 g / 23.0 g/mol = 1.52 mol
From the balanced equation, 2 moles of Na produces 1 mole of H₂. Therefore, 1.52 moles of Na produces:
1.52 mol Na × (1 mol H₂/2 mol Na) = 0.76 mol H₂
Finally, we can calculate the mass of H₂ produced using its molar mass:
molar mass of H₂ = 2.02 g/mol
mass of H₂ = moles of H₂ × molar mass of H₂ = 0.76 mol × 2.02 g/mol ≈ 1.54 g
Therefore, the mass of H₂ that can be produced from 35.0 g of Na is approximately 1.54 g. Answer: 1.54 g H₂