Glucose, C6H12O6, is best described as a(n) ______. compound. Which of the following is NOT a property of water? It is denser when frozen than when liquid.
Answers
a. Glucose, C6H12O6, is best described as a carbohydrate compound.
b. The statement that is NOT a property of water is "It is denser when frozen than when liquid." Option 1 is the correct answer.
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen atoms in a ratio of approximately 1:2:1.
In fact, water is less dense when frozen than liquid, which is why ice floats on liquid water.
This is due to the unique property of water in which its molecules form a crystal lattice structure when frozen, which causes them to be more spread out and less dense than in the liquid state.
This property is important in aquatic ecosystems as it allows ice to float on top of bodies of water, preventing them from freezing solid and allowing life to continue below the surface.
Learn more about glucose at
https://brainly.com/question/30548064
#SPJ4
The question is -
Answer the following questions -
a. Glucose, C6H12O6, is best described as a(n) ______. compound.
b. Which of the following is NOT a property of water?
Options are -
1. It is denser when frozen than when liquid.
2. It is denser when gaseous than when liquid.
3. It is lighter when frozen than when liquid.
a. Glucose, C6H12O6, is best described as a carbohydrate compound.
b. The statement that is NOT a property of water is "It is denser when frozen than when liquid." Option 1 is the correct answer.
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen atoms in a ratio of approximately 1:2:1.
In fact, water is less dense when frozen than liquid, which is why ice floats on liquid water.
This is due to the unique property of water in which its molecules form a crystal lattice structure when frozen, which causes them to be more spread out and less dense than in the liquid state.
This property is important in aquatic ecosystems as it allows ice to float on top of bodies of water, preventing them from freezing solid and allowing life to continue below the surface.
Learn more about glucose at
brainly.com/question/30548064
#SPJ4
Related Questions
Which element does the electron configuration 1s2s2p2 represent?
Answers
Answer:
Explanation:
Carbon
According to one acid-base theory a molecule acts as an acid when the molecule
Answers
mercury, rather than water, is used as a liquid in barometers because:
Answers
Mercury is used as a liquid in barometers because it has a high density, which means it can accurately measure small changes in atmospheric pressure.
Additionally, mercury is less affected by temperature changes compared to water, which can expand or contract with changes in temperature, affecting the accuracy of the measurement. Mercury also has a low vapor pressure, which means it will not evaporate easily and will remain in the barometer for a long time.
However, it is important to note that mercury is toxic and can be harmful to both humans and the environment if it is not handled and disposed of properly.
Mercury is used in barometers because it has several advantageous properties. Firstly, its high density allows for a shorter column, making the device more compact. Additionally, mercury's low vapor pressure reduces evaporation, ensuring accurate pressure measurements. Moreover, mercury's high surface tension prevents it from sticking to the barometer's glass walls, leading to precise readings. Finally, it does not freeze at standard atmospheric temperatures, allowing for consistent functionality. These properties make mercury a more suitable choice than water for measuring atmospheric pressure in barometers.
To know about Mercury :
https://brainly.com/question/19940784
#SPJ11
100 POINTS PLEASE HELP (PHOTOS INCLUDED)


Answers
Answer:
Explanation:
3.
Knowns: 100mL of solution; concentration of 0.7M
Unknown: number of moles
Equation: number of moles = volume * concentration
Plug and Chug: number of moles = 100/1000 * 0.7 = 0.07 mole
Final Answer: 0.07mole
2.
Knowns: 5.50L of solution; concentration of 0.400M
Unknown: number of moles
Equation: number of moles = volume * concentration
Plug and Chug: number of moles = 5.5 * 0.4 = 2.20 mole
Final Answer: 2.20 mole
Moles of solute be x
#1
Volume of solution=100mL=0.1mLMolarity=0.7M\(\\ \rm\Rrightarrow Molarity=\dfrac{Moles\:of\:solute}{Volume\:of\'solution}\)
\(\\ \rm\Rrightarrow 0.7=\dfrac{x}{0.1}\)
\(\\ \rm\Rrightarrow x=0.1(0.7)\)
\(\\ \rm\Rrightarrow x=0.07mol\)
#2
Same like before
Volume of solution=5.5LMolarity=0.4M\(\\ \rm\Rrightarrow \dfrac{x}{5.5}=0.4\)
\(\\ \rm\Rrightarrow x=5.5(0.4)\)
\(\\ \rm\Rrightarrow x=2.20mol\)
Need help with my work chemistry assignment grade 12 college work please

Answers
Answer:
A)0.450 mol N2
B)294 g NH4 NO3
Explanation:Step by step! make sure when you see underneath the multipication eqaution that we are deviding!The arrows is what we are dividing
A)36.0gN H4N O3×1 molN H4NO3×2mol N2
↑ 1 ↑ 80.05gNH4NO3 ↑2mol N H4N O3
B)7.35molH2O×2mol N H4 N O3×80.05gN H4 N O3
↑ 1 ↑ 4molH2O ↑ 1mol N H4N O3
5) 2 and for the how many moles it is 10.9
Hope this helps!
An ionic substance is formed between a positively charged cation and a negatively charged anion. Ionic substances are generally formed from metal and nonmetal atoms with the metal atoms forming the cations and the nonmetal atoms forming the anions. Which of the following substances are ionic? Check all that apply. Check all that apply. AlCl3 MgI2 C2H5OH NH3
Answers
Answer:
Which of the following substances are ionic?
Check all that apply.
AlCl3
MgI2
C2H5OH
NH3
Explanation:
An ionic substance is formed between a positively charged cation and a negatively charged anion.
Ionic substances are generally formed from metal and nonmetal atoms with the metal atoms forming the cations and the nonmetal atoms forming the anions.
Among the given substances,
MgI2 is an ionic substance.
Since Mg is the metal atom. It forms a cation.
I is a nonmetal atom. It forms anion.
Hence,
MgI2 is an ionic substance.
Though AlCl3 looks like an ionic substance it is a covalent compound.
This is due to the high polarizability of \(Al^3+\) ion.
The remaining two compounds do not contain any metal atoms.
So, ethanol and NH3 are not ionic compounds.
Answer:
MgI2
A olvent i found to be 50. 0% oxygen, 37. 5% carbon, and 12. 5% hydrogen. What i the empirical formula of thi olvent
Answers
The empirical formula of the solvent is CH4.
Relative number of atoms
Of H= 25/1 = 25
Of C= 75/12 = 6.25
What is a solvent?
Solvents are a heterogeneous group of structurally different chemicals that can be used to dilute, dissolve, or disperse other compounds. The ability of a solvent to dissolve another molecule depends on the molecular structure and physical properties of both the solvent and the solute. Solvents can be categorized as organic or inorganic and in terms of chemical polarity. Polar solvents include water, alcohols, and other chemicals containing –OH, such as acetic acid, which have the ability to donate H+ and form hydrogen bonds. Polar solvents lacking the –OH group, including acetonitrile, dimethylformamide, and dimethylsulfoxide, are protophilic solvents and are used to dissolve less polar solutes.To know more about solvents and solutes, click the link given below:
https://brainly.com/question/17061863
#SPJ4
An ideal gas has a density of 1.17×10
−6
g/cm
3
at 1.00×10
−3
atm and 60.0
∘
C. Identify the gas. Oxygen Neon Hydrogen Chlorine Argon Nitrogen
Answers
The gas is Argon.
To identify the gas, we need to compare the number of moles calculated for each gas with the given density.
Let's calculate the number of moles for each gas and compare them:
Given:
Density = 1.17 × 10^(-6) g/cm^3
Pressure = 1.00 × 10^(-3) atm
Temperature = 60.0 °C = 60.0 + 273.15 = 333.15 K
Molar mass of Oxygen (O2) = 32.00 g/mol
Molar mass of Neon (Ne) = 20.18 g/mol
Molar mass of Hydrogen (H2) = 2.02 g/mol
Molar mass of Chlorine (Cl2) = 70.90 g/mol
Molar mass of Argon (Ar) = 39.95 g/mol
Molar mass of Nitrogen (N2) = 28.02 g/mol
For Oxygen (O2):
n = (PV) / (RT) = (1.00 × 10^(-3) atm) × (1.17 × 10^(-6) g/cm^3) / ((0.0821 L × atm/(mol × K)) × 333.15 K)
n = 5.88 × 10^(-12) mol
For Neon (Ne):
n = (PV) / (RT) = (1.00 × 10^(-3) atm) × (1.17 × 10^(-6) g/cm^3) / ((0.0821 L × atm/(mol × K)) × 333.15 K)
n = 9.26 × 10^(-12) mol
For Hydrogen (H2):
n = (PV) / (RT) = (1.00 × 10^(-3) atm) × (1.17 × 10^(-6) g/cm^3) / ((0.0821 L × atm/(mol × K)) × 333.15 K)
n = 9.26 × 10^(-11) mol
For Chlorine (Cl2):
n = (PV) / (RT) = (1.00 × 10^(-3) atm) × (1.17 × 10^(-6) g/cm^3) / ((0.0821 L × atm/(mol × K)) × 333.15 K)
n = 2.58 × 10^(-12) mol
For Argon (Ar):
n = (PV) / (RT) = (1.00 × 10^(-3) atm) × (1.17 × 10^(-6) g/cm^3) / ((0.0821 L × atm/(mol × K)) × 333.15 K)
n = 4.64 × 10^(-12) mol
For Nitrogen (N2):
n = (PV) / (RT) = (1.00 × 10^(-3) atm) × (1.17 × 10^(-6) g/cm^3) / ((0.0821 L × atm/(mol × K)) × 333.15 K)
n = 6.45 × 10^(-12) mol
Comparing the number of moles calculated for each gas with the given density, we find that the gas with the closest value is Argon (Ar). Therefore, the gas is Argon.
Learn more about Argon from the given link
https://brainly.com/question/23949935
#SPJ11
How many grams are in 88.2 moles of magnesium?
Answers
Answer:
21409
Explanation:
Please give the brainliest.
Mosquito repellent is sprayed on one arm and the other arm is not sprayed. The number of mosquito bites is counted after 2 hours
Whats the independent and dependent variable?
Answers
Draw diagrams to show the arrangement of particles in a
Salid a liquid, and a gas
Answers
Answer:
solid(particles are rightly packed)
liquid(particles are losely packed)
gas(particles move freely)
Explanation:
there u go, hope it helps

True or False?
Mendeleev organized elements in his periodic table in order of increasing atomic number.
Answers
Answer:
True
Explanation:
because hydrogen is the first element and the atomic mass is 1 and after that the atomic number is kept increase
Which fatty acid does not pack tightly together due to a bend in the structure?
Answers
The type of fatty acid which do not bind together due to the bend in the structure ae unsaturated fatty acids which have branched chain structures.
The unsaturated fatty acids are those which have one or more carbon-carbon double bonds. For example: palmitoleic acid, oleic acid are considered as unsaturated fatty acids. The unsaturated fatty acids cannot be tightly packed due to which it makes them easier to pass more fluidly through the body.
The monounsaturated fatty acids are also liquid at room temperature due to the bend structure. On the other hand, saturated fatty acids can be easily packed as they are generally straight chain compounds.
Learn more about unsaturated fatty acids at:
brainly.com/question/11388955
#SPJ4
A scientist is studying the impact of a new manufacturing plant on the local ocean ecosystem. How could she best monitor the impact of the manufacturing plant on populations in this ecosystem?
Answers
Answer:
A. Wild life survey
Explanation:
The only answer that involved observing the wild life
What is the name of this compound? H single bonded to N with a pair of electron dots above and a single bond to H below, single bonded to the right to C with H above and below, and another C with H above, below, and right.
Answers
Answer:
Ethanamine (also known as ethylamine)
Explanation:
The compound that is requested by the question is ethanamine. Its trivial name is ethylamine.
It is a compound that contained the ethyl moiety (CH3CH2-) as well as the amine moiety (-NH2).
Ethanamine has a structure that can easily be determined by the statements in the question.
The structure of ethanamine is shown in the image attached.

Answer:
What is the name of this compound?
H H
│ │
H ── N ── C ── C ── H
│ │ │
H H H
❌ A) ethanal
✔️B) ethylamine❌ C) ethanoic acid
❌ D) methyl ethanoate
I Took Question Test And Have Day.
Assume you need to achieve a nitrogen concentration of 0.52 wt% at a position 5 mm into an iron-nitrogen alloy that initially contains 0.08 wt% N. The surface concentration is to be maintained at 1.00 wt% N, and the treatment is to be conducted at 1,100 K. (D. = 9.10E-05 m2/s and Qd = 168 kJ/mol) 25) Find the diffusion coefficient at 1,100 K if k=8.31 a) 8.91x10-12 m2/s b) 9.49x10-13 m²/s c) 7.44x10-11 m2/s d) 4.39x10-12 m2/s e) NoA
Answers
The diffusion coefficient is 4.39x10-12 m2/s.
Given information;
Initial nitrogen concentration, c₀ = 0.08 wt %
Nitrogen concentration to be achieved, cₙ = 0.52 wt %
Diffusion coefficient, D = 9.10E-05 m²/s
Temperature, T = 1100 K
Activation energy, Qd = 168 kJ/mol
Gas constant, R = 8.31 J/mol K
To find;
Diffusion coefficient at 1100 K using Arrhenius equation;
The Arrhenius equation for diffusion coefficient is given as;
D = D₀ exp(-Qd / R T)
where; D₀ is the diffusion coefficient at an infinite temperature.
Substituting the given values of D, Qd, R, and T into the equation above;
D = 9.10E-05 m²/s
Qd = 168 kJ/mol
R = 8.31 J/mol
KT = 1100 K
At 1100 K, the value of kT is;
kT = R T
= 8.31 J/mol K x 1100 K
= 9141 J/mol
Multiplying by Avogadro's number to get the value in J;
9141 J/mol x (6.022 x 10²³) / (1 mol) = 5.50 x 10²⁹ J-1
= 5.50 x 10²⁹ m²/kg
Multiplying by the Boltzmann constant to get the value in m²/s;
K = 1.38 x 10⁻²³ J/KD₀ can now be obtained by rearranging the Arrhenius equation as;
D₀ = D / exp(-Qd / R T)
Substituting the values into the equation;
D₀ = 9.10E-05 m²/s / exp(-168 x 10³ J/mol / 8.31 J/mol K x 1100 K)D₀
= 9.10E-05 m²/s / exp(-21.36)D₀
= 9.10E-05 m²/s / 1.29E-09D₀
= 7.05E-04 m²/s
Therefore, the diffusion coefficient at 1,100 K if k = 8.31 is;
D = D₀ exp(-Qd / R T)D
= 7.05E-04 m²/s exp(-Qd / R T)D
= 7.05E-04 m²/s exp(-168 x 10³ J/mol / 8.31 J/mol K x 1100 K)
D = 7.05E-04 m²/s exp(-21.36)D
= 4.39 x 10⁻¹² m²/s
Therefore, the correct option is 4.39x10-12 m2/s.
Learn more about Concentration from the given link :
https://brainly.com/question/17206790
#SPJ11
when electrophoretic separations are done based on molecular weight, the distance that a molecule moves can be graphed as a straight line when compared to:
Answers
The distance that a molecule moves can be graphed as a straight line when compared to: the log of the molecular weight of the proteins.
Correct option is D. the log of the molecular weight of the proteins.
This is known as the "Laws of Electrophoresis", which states that the rate at which different sized molecules move through a gel has an inverse relationship to the molecular weight of the particles being separated. The smaller the size of the particles, the faster they will move, and the greater the charge benefit due to the Coulombic effect.
The ultimate result is that the greater the molecular weight of the molecule being separated, the longer the distance it will travel, thus forming a straight line when graphed on a logarithmic scale. This allows one to calibrate their electrophoresis gels and to accurately estimate the molecular weight of a given set of samples.
This process is a highly effective tool used to isolate and identify different molecules, making it a key technique in many fields.
Correct option is D. the log of the molecular weight of the proteins.
know more about molecular weight here
https://brainly.com/question/20380323#
#SPJ11
Complete question is:
When electrophoretic separations are done based on molecular weight, the distance that a molecule moves can be graphed as a straight line when compared to:
A. The molecular weight of the proteins
B. MW /2
C. None of these the negative of the molecular weight of the proteins
D. the log of the molecular weight of the proteins
a balloon is filled to a volume of 2.00 l with 4.00 moles of gas at 25 °c. with pressure and temperature held constant, what will be the volume of the balloon if 0.85 moles of gas are released?
Answers
The volume of the balloon will be 2.70 L after 0.85 moles of gas are released, assuming that the pressure and temperature are held constant.
The ideal gas law can be used to solve this problem: PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature in Kelvin. Since the pressure and temperature are held constant, we can use the formula PV = constant to relate the initial and final volumes of the balloon.
P1V1 = P2V2
P2 = P1
V2 = P1V1 / P2
V2 = (49.05 atm)(2.00 L) / (3.15 mol)(0.0821 L•atm/mol•K)(298 K)
V2 = 2.70 L
To know more about pressure visit:-
https://brainly.com/question/12971272
#SPJ11
Please answer and thank you

Answers
Answer:
It's A.
Atoms that have the same number of valence electrons in their outer shell have similar properties and belong to the same family of elements.
an element Y has 13 protons . with reference on Y answer these questions •
•state the no of electrons and neutrons.
•what is the mass no
•how many valence electrons are there in y
•is it a metal\ non metal or noble gas
•what is its valency
•
Answers
Explanation:
Y has 13 protons and we know that Aluminium (Al) too has 13 protons.
So , element Y = Aluminium
• In an element , no. of protons = no. of electrons. So , Y has 13 electrons.
• Mass no. of Y (Aluminium) = 27.
• Also we know that ,
Mass number = no. of protons + no. of neutrons
So,
No. of neutrons = 27 - 13 = 14
• Valence electrons are the electrons which are present in the outermost shell of an element.
Electronic Configuration of Y = 2,8,3
So, Y has 3 valence electrons.
• We know that Y is Aluminium & Aluminium is a metal. So , Y is a metal.
• Valency of Y = +3
A 60.00g sample of tetraethyl lead, a gasoline additive, is found to contain 38.43g lead, 17.83g carbon, and 3.74g hydrogen. Find its empirical formula.
Answers
In a 60g sample of tetraethyl-lead, a gasoline is addictive, is found to contain 38.43g lead, 17.83g carbon and 3.74g hydrogen, its empirical formula is PbC₆H₂₀.
What is empirical formula ?The term Empirical formula is defined as the chemical formula of a compound that gives the ratios of the elements present in the compound but not the actual numbers or arrangement of atoms.
The percentage mass of Pb = 38.43/60 × 100
= 64.05 %
The percentage mass of C = 17.83/60 × 100
= 29.71%
The percentage mass of H = 3.74/60 × 100
= 6.23%
Now,
Pb → 64.05/207
= 0.3094
C ⇾ 29.71/12
= 2.475
H ⇾ 6.23/1
= 6.23
Therefore, the ratio is as follows:
Pb : C : H = 1 : 8 : 20
Thus, empirical formula is PbC₆H₂₀.
To learn more about an empirical formula, follow the link;
https://brainly.com/question/14044066
#SPJ9
6 limiting your answer to cycloalkanes and ignoring stereoisomers, how many c6h12 constitutional isomers are there?
Answers
When considering only cycloalkanes and ignoring stereoisomers, there are five constitutional isomers for the formula C6H12.
The constitutional isomers for C6H12 can be determined by considering different arrangements of carbon atoms in a cyclic structure. The possible constitutional isomers for C6H12 are as follows:
Cyclohexane: It consists of a six-membered carbon ring with all single bonds.
Methylcyclopentane: It contains a five-membered carbon ring with an additional methyl (CH3) group attached.
Ethylcyclobutane: It consists of a four-membered carbon ring with an additional ethyl (C2H5) group attached.
Dimethylcyclopropane: It contains a three-membered carbon ring with two additional methyl (CH3) groups attached.
Trimethylcyclopropane: It consists of a three-membered carbon ring with three additional methyl (CH3) groups attached.
These five constitutional isomers represent all the different ways the C6H12 formula can be arranged in cycloalkane structures, disregarding stereochemistry.
To know more about constitutional isomers, click here:
https://brainly.com/question/30556576
#SPJ11
Describe the plum pudding model
Answers
Answer:
The plum pudding model is one of several historical scientific models of the atom. ... The plum pudding model has electrons surrounded by a volume of positive charge, like negatively-charged "plums" embedded in a positively-charged "pudding".
The mass of a mole of a substance can be found using the ____ of the substance.
Answers
Answer:
mass
Explanation:
Answer:
mass mass mass mass mass
Question 2
A group of students conducted an investigation in which they observed chemical and physical properties of a sheet of paper.
Which is a chemical property of a sheet of paper ? HURRY PLEASE!

Answers
Answer:
A
Explanation:
Answer:a
Explanation:cuz ik this
1)A circuit is switched on for 60s with a current of 4A. How much charge flowed?
Answers
Charge flowed : 240 Coulombs
Further explanationGiven
t = time = 60 s
I = current = 4 A
Required
Charge
Solution
General formula :
Q = I x t
Q = charge (electricity), C
Input the value :
Q = 4 x 60
Q = 240 Coulombs
if you mix 40g of hcl and 1g of mg, is the pH less than 7, neutral, or more than 7
Answers
Describe the arrangement of ions in a crystal lattice.
Answers
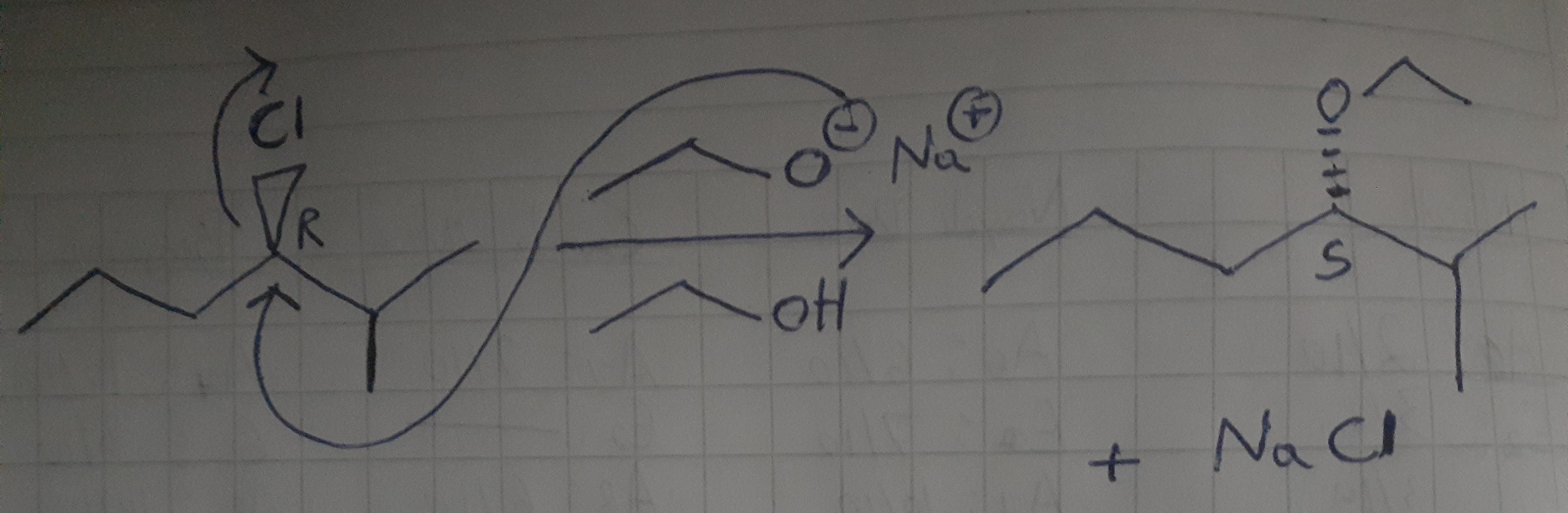
Part b (r)–3-chloro-2-methylhexane will undergo a nucleophilic substitution reaction in the presence of sodium ethoxide and ethanol. complete the mechanism for the sn2 reaction and draw the products of the reaction. draw all missing reactants and/or products in the appropriate boxes by placing atoms on the grid and connecting them with bonds and including charges where needed. indicate the mechanism by drawing the electron-flow arrows on the molecules. arrows should start on an atom or a bond and should end on an atom, bond, or where a new bond should be created.
Answers
Answer:
See answer below
Explanation:
To understand this, we need to make the reaction involved with the given configuration. Configuration R means that the substituents of the molecule, the priority order goes clockwise (From heaviest to lightest) so, if an Sn2 is ocurring in the reaction this means that the nucleophile, which in this case, is the sodium ethoxide will attack the molecule in the opposite side of the leaving group (which is the chlorine).
Also when this occurs, as Sn2 is a bimolecular reaction and is held in one step, it occurs an inversion in the configuration of the product. So, it's the innitial reactant was R, then the final product will be S.
The mechanism and product can be watched in the attached picture
Hope this helps.
A condenser is used to convert 10 kg/s of vapor with enthalpy of 2600 kJ/kg to liquid. What is the enthalpy of the liquid coming out of the condenser if the rate of heat transfer within the condenser is 25 MW
Answers
The enthalpy of the liquid coming out of the condenser is 2600 kJ/kg.
In a condenser, vapor is converted to liquid by transferring heat out of the vapor. In this case, the vapor has an enthalpy of 2600 kJ/kg. The rate of heat transfer within the condenser is given as 25 MW.
To determine the enthalpy of the liquid coming out of the condenser, we need to consider the conservation of energy. The heat transfer within the condenser is equal to the change in enthalpy of the vapor as it condenses. Since the vapor is being converted to liquid, the enthalpy of the liquid coming out will be the same as the initial enthalpy of the vapor, which is 2600 kJ/kg.
This means that the enthalpy of the liquid coming out of the condenser is 2600 kJ/kg.
Learn more about condenser
brainly.com/question/1287204
#SPJ11