Giving away 20 points and brainlest, please help
What type of transport does not require energy to move molecules across the plasma membrane?
A. bilayer
B. active
C. passive
D. concentration gradient
Answers
Answer:
A
Explanation:
Answer:
I don’t think that A is the correct answer, I believe that C may be the correct answer
Explanation:
Related Questions
which term is defined as repeating units in an organic compound?
Answers
How many carbon atoms are in 1.25 mol of silver acetate?
Answers
The number of carbon atoms present in 1.25 mol of silver acetate is 1.875 × 10²³.
What is Avogadro's number?Avogadro's number is define as the number of atoms of any substance present in one mole of that substance, and it is equal to 6.022 × 10²³, i.e.
1 mole = 6.022 × 10²³ atoms/mol
Given moles of silver acetate = 1.25 moles
Number of atoms of silver acetate in 1.25 moles = 1.25 × 6.022 × 10²³ = 7.5×10²³
Molecular formula of silver acetate is CH₃CO₂Ag, means in this molecule total 8 atoms are present so number of carbon atom will be calculated as:
Carbon atoms = (2× 7.5 × 10²³) / 8 = 1.875 × 10²³ atoms [here we multiply by 2 because 2 atoms of carbon is present in olecule]
Hence required atoms of carbon is 1.875 × 10²³.
To know more about Avogadro's number, visit the below link:
https://brainly.com/question/1513182
What are tides and what causes them? Your response must discuss high tide, low tide, spring tide, and neap tides.
Answers
Answer:
Tides are the rise and fall of sea levels caused by the combined effects of gravitational forces exerted by the Moon and the Sun, plus the rotation of the Earth. Tides are caused by the gravitational pull of the moon and sun. High and Low tides are caused by the moon. Spring tides occur twice each lunar month all year long without regard to the season. Neap tides, which also occur twice a month, happen when the sun and moon are at right angles to each other.
Explanation:
Hope this helped! :D
Give an example of how knowledge of physical properties of matter can be used in everyday life
Answers
Understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
Knowledge of physical properties of matter is extremely important in everyday life as it helps us understand the nature of substances we come into contact with. One example is the use of boiling points in cooking. Different substances have different boiling points which determine the temperature at which they boil. This information is crucial in determining cooking times and ensuring that food is cooked properly.
For instance, water boils at 100 degrees Celsius, while sugar syrup boils at a much higher temperature. If the wrong temperature is used, food may be undercooked or overcooked, leading to undesired outcomes. Knowledge of physical properties also helps in choosing the right materials for different purposes, such as choosing heat-resistant materials for cooking.
In conclusion, understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
To know more about matter visit:
brainly.com/question/28487167
#SPJ11
how does molecule movement in solids and liquids differ
Answers
Given the equilibrium system at 25°C:NH4Cl(s) = NH(aq) + Cl(aq)(AH = +3.5 kcal/mole)Which change will shift the equilibrium to the right?
Answers
Explanation
NH4Cl(s) = NH4+(aq) + Cl-(aq) AH = +3.5 kcal/mole
We have some changes that shift the equilibrium to the right, according to Le Châtelier's principle.
I will write some of them:
By Adding amount of NH4Cl or by increasing the temperature because according to the heat of the reaction this is an endothermic reaction.
Answer: Increasing the temperature of the system.
The pitch of a sound is how high or low it is. The higher the frequency of a sound wave is, the higher its pitch. Each of the waves below represents a sound wave. Put the waves in order from lowest to highest pitch

Answers
The waves in order from lowest to highest pitch : picture 1,2,3,4
Further explanationWavelength : from the crest to the crest of the next wave or the trough to the trough
Frequency (f): number of waves in one second
Can be formulated :
\(\tt v=\lambda\times f\\\\f=\dfrac{v}{\lambda}\)
So the frequency is inversely proportional to the wavelength
The smaller the wavelength the greater the frequency and the greater the pitch
From the picture above (transverse wave), we determine the wavelength that is formed: ⇒1 wavelength =one crest (peak) + trough (base)
wavelength = distance : wave(distance = 4)
\(\tt 1\rightarrow 1\dfrac{1}{2}~wave\rightarrow \lambda=\dfrac{4}{1\frac{1}{2} }=2.67 \\\\2\rightarrow 2~wave\rightarrow \lambda=\dfrac{4}{2}=2 \\\\3\rightarrow 2\dfrac{1}{2}~wave\rightarrow \lambda=\dfrac{4}{2\frac{1}{2} }=1.6\\\\4\rightarrow 3~wave\rightarrow \lambda=\dfrac{4}{3}=1.33\)
So picture 1 has the largest wavelength, so it has the smallest frequency and the smallest pitch
Answer:
The pitch of a sound is how high or low it is. The higher the frequency of a sound wave is, the higher its pitch.
Each of the waves below represents a sound wave. Put the waves in order from lowest to highest pitch.
Explanation:
Which of the following is an example of a heterogenous mixture? O soy souce O lemonade O honey O trail mix
Answers
Answer:
soysauce bro
Explanation:
Answer:
trail mix
Explanation:
a heterogeneous mixture is a mixture with non-uniform composition. two or more of the mixture's components must be separate for the mixture to be heterogeneous.
. describe how the ph of a solution relates to the hydrogen ion concentration. does a solution at ph 1 have more or less hydrogen ions than a solution at ph 4?
Answers
A solution at pH 1 has more hydrogen ions than a solution at pH 4. The pH of a solution refers to the hydrogen ion concentration.
The concentration of hydrogen ions and the pH of a solution are inversely proportional. This means that the higher the hydrogen ion concentration, the lower the pH, and vice versa.
A solution at pH 1 will have more hydrogen ions than a solution at pH 4.The pH of a solution is defined as the negative logarithm of the hydrogen ion concentration. The equation for calculating the pH of a solution is given as follows:
\($$pH = -\log_{10}[H^+]$$\)
In this equation, [H⁺] is the hydrogen ion concentration in moles per liter (mol/L) of solution. A change of 1 pH unit corresponds to a 10-fold change in the hydrogen ion concentration.
Therefore, if a solution has a pH of 1, it has a hydrogen ion concentration of 0.1 mol/L.
If a solution has a pH of 4, it has a hydrogen ion concentration of 0.0001 mol/L. Thus, a solution at pH 1 has more hydrogen ions than a solution at pH 4.
To know more about pH, refer
https://brainly.com/question/15265711
#SPJ11
No spam or leaks
Although the volume is set the shape and structure of the cup is up to you (for example your cup can be tall and skinny with a handle or short and wide without a handle) Think about the cup's intended use, and determine at least two criteria for the shape of the cup and the location of its two compartments.
( this writing question)
Answers
When designing a cup of a fixed volume, the designer must be guided by the intended use of the cup.
What is a cup?A cup is a hollow object that is primarily used to drink water. Cups are used in every home. Being a solid shape, the volume of the cup is fixed.
However, the shape and structure of the cup depends on its intended use. Hence, when designing a cup of a fixed volume, the designer must be guided by the intended use of the cup.
Learn more about volume of solid shapes:https://brainly.com/question/1578538
what would you observe if sodium and potassium are placed in hot water.
Answers
Answer:
Sodium reacts more quickly, generating enough heat to melt itself and to occasionally ignite the hydrogen gas, producing a yellow-orange flame characteristic of sodium. The potassium reacts violently, immediately bursting into a flame which has the characteristic violet color of potassium.
Explanation:
me of an Irregular Solid
Use the image to determine the volume of the rock.
Initial volume:
mL
Final volume:
Volume of rock: 20
mL
cm³
Answers
The volume of the irregular solid from the image that have been shown is 12 mL
How do you determine the volume of an irregular solid?To obtain the volume of the irregular solid;
a. Measure the initial volume of water in the cylinder.
b. Carefully lower the irregular solid into the water, ensuring it is fully submerged without trapping any air bubbles.
c. Record the new volume of water in the cylinder.
d. The volume of the irregular solid is equal to the difference between the final and initial water volumes.
The volume is;
32 mL - 20 mL = 12 mL
Learn more about irregular solid:https://brainly.com/question/31829216
#SPJ1

For the following reaction, determine which way the equilibrium would shift, toward the products or toward the reactants, given the proposed change. Assume the reaction is endothermic.
A+ B â â C+ D
Part A: If D was removed
Part B: If more B was added to the reaction
Part C: If the reaction was heated
Answers
Option A: if D was removed, the equilibrium would have shifted towards the product as D lies on the product side of the equation.
An equilibrium may be stressed in a number of ways. One method is to change the reactant or product of a chemical process when it reaches equilibrium. To lessen the stress, adding more product causes the equilibrium to switch to reactants. If a reactant or product is taken away, the equilibrium changes to produce more of the taken away reactant or product, respectively.
The chemical process is no longer at equilibrium when we stress it, and it begins to move back towards equilibrium in a way that reduces the stress. The formal formulation is termed Le Chatelier's principle: If an equilibrium is stressed, then the reaction moves to decrease the stress.
To know more about equilibrium, refer:
https://brainly.com/question/26745669
#SPJ4
Correct question is:
For the following reaction, determine which way the equilibrium would shift, toward the products or toward the reactants, given the proposed change. Assume the reaction is endothermic.
A + B → C + D
Part A: If D was removed
Part B: If more B was added to the reaction
Part C: If the reaction was heated
Summarize how the structure of organic compounds allows them to function as pigments in 2 – 3 sentences
Answers
The structure of organic compounds allows them to function as pigments through chromophore and acid or basic groups.
What is a Pigment?This is defined as a colored substance which is completely or nearly insoluble in water.
The structure of organic compounds allows them to function as pigments include chromophore and the acid or basic groups such as OH, SO3H, etc.
Read more about Pigment here vhttps://brainly.com/question/1056549
#SPJ1
1. H2SO4, sulfuric acid, contains three different types of atoms: hydrogen (H), sulfur (S), and oxygen (O). Each of these atoms represents a different . Since the three types are combined in a fixed ratio, this means that H2SO4 is a(n) molecule. 2. The smallest unit of matter that retain all of the physical properties of that type of matter is a(n) atom. 3. is anything that occupies space and/or has any substance. 4. If two or more atoms are bonded together, they form a(n) . 5. The scientific study of matter is called . 6. Within a plant, water (H2O) and carbon dioxide (CO2) can be combined (using the energy of sunlight) to produce glucose (C6H12O6) and oxygen (O2). If you were to write out this chemical reaction, water and carbon dioxide are each an example of a(n) while glucose and
Answers
Answer:
The blanks can be completed with words in the following sequence
1) elements
compound
2) atom
3) matter
4) compound
5) chemistry
6) reactants
product
Explanation:
In H2SO4, atoms of hydrogen, sulphur and oxygen represent different elements that are combined to form the compound.
The smallest indivisible unit of matter that still retains all the properties of matter is an atom. Matter is anything that has weight and occupy space.
Atoms combine in fixed ratios to form compounds. Chemistry studies matter scientifically and pays specific attention to the changes that matter undergoes.
Considering the reaction of photosynthesis, water and carbon dioxide combine to give glucose so the water and carbon dioxide are reactants while the glucose is the product of the reaction.
The electron configuration of aluminum, atomic number 13, is [Ne] 3s2 3p1. Aluminum is in Period.
Answers
Aluminum is in Period 3 because its electron configuration, [Ne] 3s2 3p1, indicates that its highest energy level is the third shell, corresponding to Period 3 in the periodic table.
The electron configuration of aluminum, with atomic number 13, is [Ne] 3s2 3p1. This indicates that aluminum has a total of 13 electrons distributed among its energy levels. The [Ne] represents the noble gas neon, which has the electron configuration 1s2 2s2 2p6. This noble gas configuration is used to represent the filled inner electron shells of aluminum. The remaining electron configuration, 3s2 3p1, shows that aluminum has two electrons in the 3s orbital and one electron in the 3p orbital. This arrangement of electrons follows the Aufbau principle, which states that electrons fill the lowest energy orbitals first before moving to higher energy orbitals. The period of an element in the periodic table corresponds to the highest principal energy level (shell) in its electron configuration. Since aluminum's highest principal energy level is the third shell (3s and 3p orbitals), it is located in Period 3 of the periodic table.
learn more about periodic table here:
https://brainly.com/question/28747247
#SPJ11
For the following unbalanced chemical equation, suppose that exactly 5.00 g of reach
reactant is taken, calculate the theoretical yield of solid silver.
AgNO3 (aq) + Al (s) --> Ag (s) + AI(NO3)3 (aq)
help me, please!
Answers
The theoretical yield of solid silver in this reaction, starting with exactly 5.00 g of AgNO3, is 1.06 g Ag. This assumes perfect reaction conditions and complete conversion of reactants to products, which may not always be the case in reality.
To calculate the theoretical yield of solid silver in this reaction, we first need to balance the chemical equation:
3AgNO3 (aq) + Al (s) --> 3Ag (s) + Al(NO3)3 (aq)
Now we can see that the stoichiometry of the reaction is 3:1 for AgNO3 to Ag. This means that for every 3 moles of AgNO3 that react, 1 mole of Ag will be produced.
To calculate the theoretical yield, we need to convert 5.00 g of AgNO3 to moles. The molar mass of AgNO3 is 169.87 g/mol, so:
5.00 g AgNO3 x (1 mol AgNO3 / 169.87 g AgNO3) = 0.0294 mol AgNO3
since the stoichiometry of the reaction is 3:1 for AgNO3 to Ag, we can calculate the number of moles of Ag that would be produced:
0.0294 mol AgNO3 x (1 mol Ag / 3 mol AgNO3) = 0.00980 mol Ag
Finally, we can convert the moles of Ag to grams using the molar mass of Ag, which is 107.87 g/mol:
0.00980 mol Ag x (107.87 g Ag / 1 mol Ag) = 1.06 g Ag (rounded to two significant figures)
To know more about theoretical yield visit:-
https://brainly.com/question/30700754
#SPJ11
b) 25.8 g C12H24012 is burned in an experiment, how many grams of carbon
dioxide is produced?
Answers
Following C12H24012's complete combustion, the following occurs:
C12H24012 + 19O2 → 12CO2 + 24H2O
The amount of carbon dioxide produced is 12 grams.
What is the mass conservation law?
The Law of Conservation of Mass, which states that mass neither creates nor destroys in a chemical reaction, is used in this question.
Steps in this instance are listed below.
1. Calculate C12H24012's molecular weight:
In order to determine the number of moles, divide the given mass of 25.8 g by the molar mass of C12H24012, which is 264 g/mol:
264 g/mol/25.8 g C12H24012 = 0.097 mol
2. Calculate the required oxygen moles:
There are 19 moles of oxygen needed for every mole of C12H24012. In order to determine the necessary amount of oxygen, multiply 0.097 mol by 19:
19 mol O2/1 mol C12H24012 x 0.097 mol C12H24012 = 1.833 mol O2
To learn more about Law of Conservation of Mass refer :
https://brainly.com/question/27891057
#SPJ1
M → Mt+e
Has M lost or gained an electron?
Answers
Answer:
M has lost an electron, one electron, and given it to Mt
Explanation:
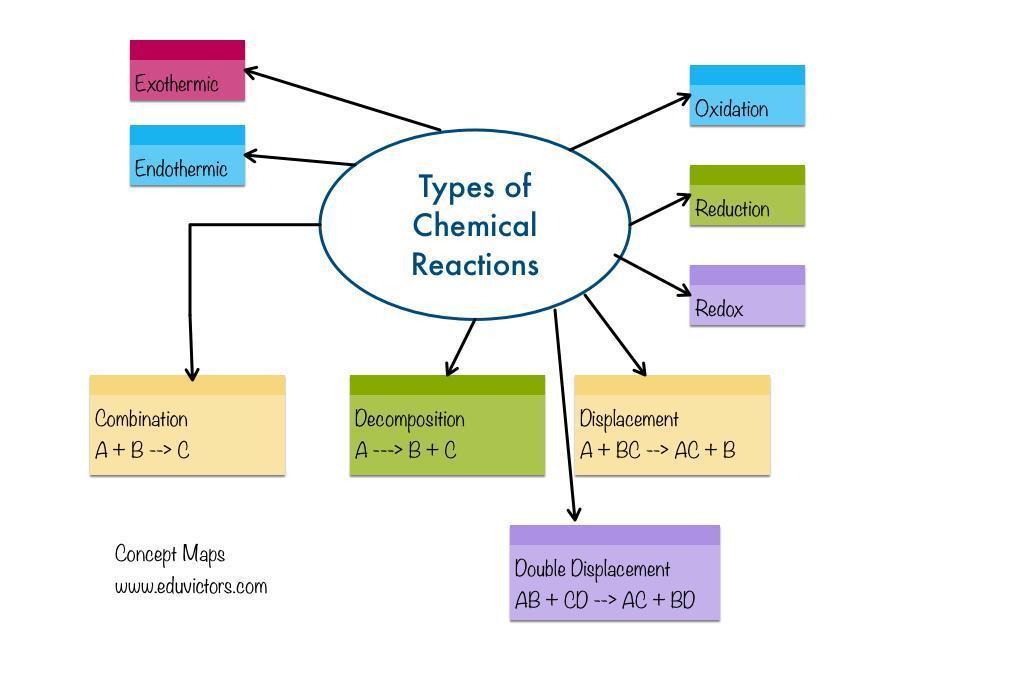
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
How many bromide ions are there in 2.00g of MgBr2?
Answers
There are 1.31 x 1022 bromide ions in 2.00 g of \(MgBr_2\).
The chemical formula of magnesium bromide (\(MgBr_2\)) contains one magnesium ion (\(Mg2^+\)) and two bromide ions (Br-). To find the number of bromide ions in 2.00 g of \(MgBr_2\), we need to use the molar mass of \(MgBr_2\) to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound, then use the stoichiometry of the chemical formula to determine the number of bromide ions present. First, we need to calculate the molar mass of \(MgBr_2\). The molar mass of \(MgBr_2\) is equal to the sum of the atomic masses of magnesium (Mg) and two bromine (Br) atoms. The atomic mass of Mg is 24.31 g/mol, and the atomic mass of Br is 79.90 g/mol. Molar mass of \(MgBr_2\) = 24.31 g/mol + (2 x 79.90 g/mol) = 184.11 g/mol Next, we can use the molar mass to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound: Number of moles of \(MgBr_2\) = mass of \(MgBr_2\) / molar mass of \(MgBr_2\)= 2.00 g / 184.11 g/mol
= 0.0109 mol Finally, we can use the stoichiometry of the chemical formula to determine the number of bromide ions present: Number of bromide ions = 2 x number of moles of \(MgBr_2\)
= 2 x 0.0109 mol
= 0.0218 mol Therefore, there are 0.0218 moles of bromide ions in 2.00 g of \(MgBr_2\). To convert this to the number of bromide ions, we can multiply by Avogadro's number (6.02 x 1023): Number of bromide ions = 0.0218 mol x 6.02 x 1023 ions/mol = 1.31 x 1022 ions
For more questions on bromide ions
https://brainly.com/question/29228517
#SPJ8
Select the correct hybridization for each central atom. Drag the appropriate items to their respective bins. HCN PF BrF3 SO, 2-
Answers
The correct hybridization for each central atom is:
HCN: sp hybridization.
PF3: sp3 hybridization.
BrF3: sp3d hybridization
SO2²-: sp2 hybridization
HCN: sp hybridization. The central atom carbon forms three sigma bonds with hydrogen, nitrogen, and carbon, requiring three hybrid orbitals. One electron pair is left unhybridized in a p orbital.
PF3: sp3 hybridization. The central atom phosphorus forms three sigma bonds with fluorine and one lone pair, necessitating four hybrid orbitals.
BrF3: sp3d hybridization. The central atom bromine forms three sigma bonds with fluorine and has two lone pairs. It requires five hybrid orbitals for bonding.
SO2²-: sp2 hybridization. The central atom sulfur forms two sigma bonds with oxygen and has one lone pair, requiring three hybrid orbitals. The molecule has a trigonal planar shape.
Hybridization describes the mixing of atomic orbitals to form new hybrid orbitals, allowing for the arrangement of electron pairs and bonding. The number and type of sigma bonds and lone pairs on the central atom determine its hybridization.
To learn more about hybridization here
https://brainly.com/question/32068264
#SPJ4
How is energy transferred through the water cycle?
Please help
Answers
Answer:
The water cycle is driven primarily by the energy from the sun.
liquid co2 is not stable so we usually never see it. True or false
Answers
Which three terms name a domain of life
Answers
Answer:
Bacteria, Archaea and Eukarya are the three domains of life.
10) How much 0.075 M NaCl solution can be made by diluting 450 mL of 9.0 M NaCl?
Answers
Answer:
54L
Explanation:
Solid sodium carbonate reacts with aqueous hydrochloric acid to form aqueous sodium chloride, carbon dioxide and water.
Na2CO3 + 2HCl = 2NaCl + CO2 + H2O
a. Rewrite this question to include state symbol
b. Calculate the number of moles of hydrochloric acid required
to react exactly with 4.15 g of sodium carbonate.
(A, values: C= 12.0, Na 23.0, O- 16.0, H=1.0, Cl = 35.5)
Answers
Answer:
a.
Na2CO3 (aq) + 2 HCl (aq) → H2O (l) + CO2 (g) + 2 NaCl (aq)
b.
0.0783 mols of HCl
Explanation:
Na2CO3 (aq) + 2 HCl (aq) → H2O (l) + CO2 (g) + 2 NaCl (aq)
n= 1 n= 2
Mr = 106 Mr= 36.5
m= 106g m= 73g
106 g Na2CO3 reacts with 73 g HCl
1 g Na2CO3 will react with 73/106 g HCl
4.15 g Na2CO3 will react with (73/106)× 4.15 = 2.858 g HCl
number of moles = mass/ Mr
num of moles of HCL = 2.858/36.5
= 0.07830188678
= 0.0783 mols
a. Balanced equation with state symbols:
Solid sodium carbonate (Na₂CO₃(s)) + Aqueous hydrochloric acid (2HCl(aq)) = Aqueous sodium chloride (2NaCl(aq)) + Carbon dioxide (CO₂(g)) + Water (H₂O(l))
b. 0.05 moles of HCl is required to react with 4.15 g of sodium carbonate.
To calculate the number of moles of hydrochloric acid (HCl) required to react with 4.15 g of sodium carbonate (Na₂CO₃), we first need to determine the molar mass of Na₂CO₃.
Molar mass of Na₂CO₃:
2(Na) + 1(C) + 3(O) = 2(23.0 g/mol) + 12.0 g/mol + 3(16.0 g/mol) = 46.0 g/mol + 12.0 g/mol + 48.0 g/mol = 106.0 g/mol
Next, we can use the given mass and molar mass to calculate the number of moles of Na₂CO₃:
Number of moles = Mass / Molar mass
Number of moles = 4.15 g / 106.0 g/mol ≈ 0.0391 moles
According to the balanced equation, 1 mole of Na₂CO₃ reacts with 2 moles of HCl. Therefore, the number of moles of HCl required to react with 0.0391 moles of Na₂CO₃ is:
Number of moles of HCl = 2 × 0.0391 moles ≈ 0.0782 moles
Thus, 0.0782 moles of HCl (or approximately 0.05 moles when rounded to two decimal places) are required to react exactly with 4.15 g of sodium carbonate.
To learn more about molar mass here
https://brainly.com/question/31545539
#SPJ2
What is the volume of 4.78g of O2 gas at STP?
Answers
Answer:
Explanation:
The trick here is to realize that if you know the volume of a gas at STP, you can use the fact that
1
mole of any ideal gas occupies
22.7 L
under STP conditions to calculate how many moles of gas you have in your sample.
Under STP conditions:
1 mole of an ideal gas = 22.7 L
−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−−
In your case, you know that your sample of gas occupies
2.28 L
under STP conditions, which are currently defined as a pressure of
100 kPa
and a temperature of
0
∘
C
.
This means that your sample will contain
2.28
L
⋅
molar volume of a gas at STP
1 mole gas
22.7
L
=
0.10044 moles gas
Now, the molar mass of the gas is the mass of exactly
1
mole of the gas. In your case, you know that you get
3.78 g
for every
0.10044
moles, which means that you have
1
mole
⋅
3.78 g
0.10044
moles
=
37.6 g
Since this is the mass of
1
mole of gas, you can say that the molar mass of the gas is
molar mass = 37.6 g mol
−
1
−−−−−−−−−−−−−−−−−−−−−−−
True or false all the isomers
Answers
False. Not all chemical compounds have isomers, which are molecules with the same molecular formula but different structural arrangements. Isomers only occur in certain cases where a molecule's atoms can be rearranged to create different structures with distinct properties.
The same molecular formula applies to structural isomers and other types of isomers, which are also chemical substances. It follows that these substances have the same molar mass because they share the same molecular formula.
When a substance has the same molecular formula but a different structural makeup, it is an isomer. They make up the group known as stereoisomers, or spatial isomers, which also includes optical isomers where isomers only differ in the spatial arrangement of their atoms.
To know more about Isomers visit:-
https://brainly.com/question/12796779
#SPJ11
I NEED HELP PLS ILL GIVE BRAINLY

Answers
The correct statement about Dunite is that it is formed when magma cooled slowly below the ground; Option B.
What are rocks?Rocks are large aggregates of minerals that occur in the earth's crusts as a result of the cooling ad solidification of molten magma or the deposition of the remains of dead organic matter which changes from pressure ad heat.
There are three types of rocks, and they are:
Igneous rocks - formed by the cooling of molten magma e.g. Dunite, a light yellowish-green, intrusive igneous rockSedimentary rocks - formed from the remains of dead organic matterMetamorphic rocks - are formed from changes that occur in sedimentary rocks.Learn more about igneous rocks at: https://brainly.com/question/18297174
#SPJ1