Given the following information at 25°C, Calculate ΔS° for the reaction:
N2O4(g) → 2NO2(g)
Species S° J/(mol.K)
NO2(g) 240.06
N2O4(g) 304.29
a) -175.83 J/K
b) -64.23 J/K
c) 175.83 J/K
Answers
Thus, the correct option is (a) 175.83 J/K.
Given the following information at 25°C, we have to calculate ΔS° for the reaction N2O4(g) → 2NO2(g).Solution:At 25C, the standard entropy values of N2O4 and NO2 are 304.29 J/mol K and 240.06 J/mol K respectively. ΔS° for the reaction can be calculated by the formulaΔS° = ΣS°(products) - ΣS°(reactants)In this reaction, there are two molecules of NO2, so the equation must be multiplied by 2.N2O4(g) → 2NO2(g)So,ΔS° = 2 (240.06 J/mol K) – 304.29 J/mol K= 176.83 J/K
Know more about Entropy value here:
https://brainly.com/question/24278877
#SPJ11
Related Questions
Barium sulfate, BaSO 4 VS Barium hydroxide, Ba(OH) 2 How many more oxygen atoms are represented in the formula for barium sulfate than in the formula for barium hydroxide ?
Answers
Answer:
BaSO₄ has 4 oxygens. Ba(OH)₂ has 2 oxygens.
Explanation:
The number of any one element is the coefficient times the subscript of that element. In BaSO₄, the coefficient is 1 and the subscript for oxygen is 4. In Ba(OH)₂, the coefficient is 1 and the subscript is 2. In Ba(OH)₂, the subscript is outside the () because OH⁻ is an ion and the subscript applies to both elements in the ().
Answer:
2
Explanation:
Ice at 0.0°C is mixed with 7.30 × 10^2 mL of water at 25.0°C. How much ice must melt to lower the water temperature to 0.0°C? The specific heat capacity of water is 4.186 J/(g·K). Latent heat of fusion for water is 333.7 J/g.
Answers
Approximately 35.90 grams of ice must melt to lower the water temperature to 0.0°C.
To solve this problem, we need to calculate the amount of heat that needs to be transferred from the water to the ice in order to lower the water temperature to 0.0°C.
First, let's calculate the initial heat content of the water. The specific heat capacity of water is 4.186 J/(g·K), and the mass of the water can be calculated using its density (1 g/mL) and volume (7.30 × 10^2 mL):
Mass of water = density × volume = 1 g/mL × 7.30 × 10^2 mL = 7.30 × 10^2 g
The initial heat content of the water can be calculated using the formula:
Heat content = mass × specific heat capacity × temperature change
Heat content = 7.30 × 10^2 g × 4.186 J/(g·K) × (25.0°C - 0.0°C) = 7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C
Next, we need to calculate the amount of heat that needs to be transferred from the water to the ice to lower the water temperature to 0.0°C. This heat transfer occurs during the melting of the ice.
The amount of heat required to melt the ice can be calculated using the formula:
Heat = mass of ice melted × latent heat of fusion
Let's assume that x grams of ice melts. The mass of the ice can be calculated using its density (0.92 g/mL) and volume (same as the volume of water):
Mass of ice = density × volume = 0.92 g/mL × 7.30 × 10^2 mL = 6.716 × 10^2 g
Heat = x g × 333.7 J/g
Now, we need to ensure that the heat transferred from the water to the ice is enough to lower the water temperature to 0.0°C. The heat transferred from the water to the ice is equal to the heat transferred from the water when its temperature drops to 0.0°C:
Heat content of water = Heat transferred to ice
7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C = x g × 333.7 J/g
Now, we can solve for x:
x = (7.30 × 10^2 g × 4.186 J/(g·K) × 25.0°C) / (333.7 J/g)
x ≈ 35.90 g
Therefore, approximately 35.90 grams of ice must melt to lower the water temperature to 0.0°C.
Learn more about temperature
https://brainly.com/question/27944554
#SPJ11
what is the pH of a solution with [H+] = 1.25 x 10^-10M?
Answers
pH = -log[H+]
Given [H+] = 1.25 x 10^-10 M:
pH = -log(1.25 x 10^-10)
pH = -log(1.25) + log(10^-10)
pH ≈ -9 + (-10)
pH ≈ -19
Therefore, the pH of the solution with [H+] = 1.25 x 10^-10 M is approximately -19.
Answer:
9.90
Explanation:
Given [H+] = 1.25 x 10^-10 M, we can calculate the pH using the formula:
pH = -log10([H+])
pH = -log10(1.25 x 10^-10)
Using logarithmic properties:
pH = -log10(1.25) - log10(10^-10)
Since log10(10^-10) is equal to -10:
pH = -log10(1.25) - (-10)
pH = -log10(1.25) + 10
Now, evaluating the logarithm using a calculator:
pH = -0.0969 + 10
pH = 9.9031
Therefore, the pH of the solution with [H+] = 1.25 x 10^-10 M is approximately 9.9031. Rounding it to two decimal places, the pH is approximately 9.90.
The activation energy of a certain reaction is 48.4 kJ/mol kJ/mol . At 26 âC âC , the rate constant is 0.0130sâ10.0130sâ1 . At what temperature in degrees Celsius would this reaction go twice as fast?
Answers
The temperature at which the reaction would go twice as fast is 67.2°C when the activation energy of a certain reaction is 48.4 kJ/mol kJ/mol.
The rate constant of a reaction is related to its activation energy through the Arrhenius equation, which states that k = \(Ae^{(-Ea/RT)}\), where k is the rate constant, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin.
To find the temperature at which the reaction would go twice as fast, we can use the fact that the rate constant is proportional to the reaction rate, so if we want the reaction to go twice as fast, we need to double the rate constant.
Using the Arrhenius equation, we can write:
\(k1 = Ae^{(-Ea/RT1)}\)
\(k2 = Ae^{(-Ea/RT2)}\)
where k1 is the rate constant at 26°C, k2 is the rate constant at the unknown temperature, T1 is 26°C converted to Kelvin (299 K), and T2 is the unknown temperature converted to Kelvin.
We know that we want k2 to be twice k1, so:
2k1 = k2
\(2Ae^{(-Ea/RT1)} = Ae^{(-Ea/RT2)}\)
Simplifying:
\(2 = e^{(Ea/R * (1/T2 - 1/T1))}\)
Taking the natural logarithm of both sides: \(k2 = Ae^{(-Ea/RT2)}\)
ln(2) = Ea/R * (1/T2 - 1/T1)
Rearranging:
T2 = 1/(1/T1 + (R/Ea)*ln(2))
Plugging in the values we have:
T2 = 1/(1/299 + (8.314/48.4)*ln(2))
T2 = 340.3 K
Converting back to Celsius:
T2 = 67.2°C
To learn more about activation energy click here https://brainly.com/question/28384644
#SPJ11
which type of leukocyte contains heparin, an anticoagulant?
Answers
The type of leukocyte (white blood cell) that contains heparin, an anticoagulant, is the Basophil.
Basophils are a type of granulocyte, characterized by the presence of granules in their cytoplasm. These granules contain various substances, including heparin, histamine, and other inflammatory mediators.
Heparin is an important anticoagulant that inhibits blood clotting. Basophils release heparin as part of their immune response to prevent excessive clotting and promote blood flow in areas of inflammation or injury.
Mast cells are tissue-resident cells that are similar to basophils and play a role in allergic reactions and inflammation. Both basophils and mast cells contribute to the release of heparin and other mediators in the immune response.
Learn more about Leukocyte:
https://brainly.com/question/29769387
What is an empirical formula
Answers
Answer:
a formula giving the proportions of the elements present in a compound but not the actual numbers or arrangement of atoms.
Explanation:
Answer:
Meaning of Empirical formula :- A formula giving the proportions of the elements present in a compound but not the actual numbers or arrangement of atoms.Defination of Empirical formula :- The empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S₂O₂.Explanation:
Hope this helps you XD ✌️Carry on learning !!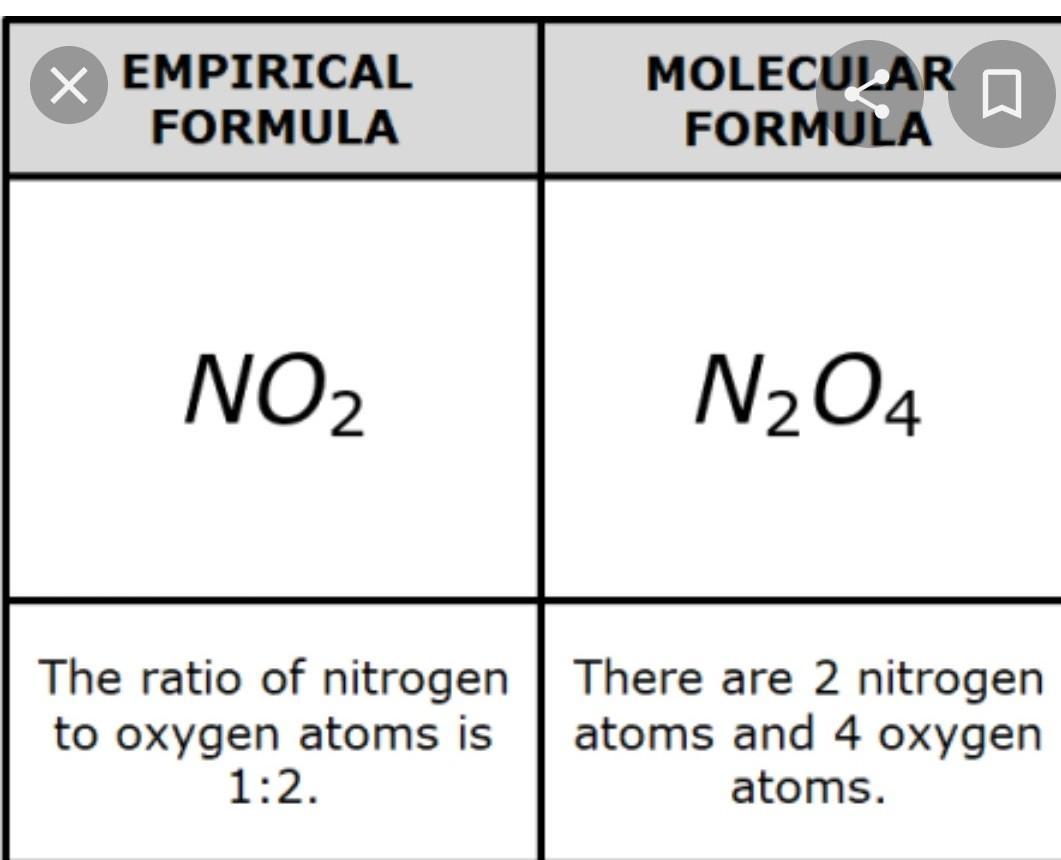


16) What is the aluminum ion concentration in a solution that is 0.646 M in aluminum sulfate
Answers
Aluminum ion has a charge of 3+, Al³⁺, and sulfate is SO₄²⁻, so the compound aluminum sulfate has to have a number of aluminum and sulfate such that the final charge is zero, so the proportion on aluminum sulfate is:
\(Al_2(SO_4)_3\)That way we have 6+ and 6-, so neutral compound.
This means that for 1 mol of aluminum sulfate, we have 2 moles of aluminum ion. The molar concentration is the number of moles of solute divided by the volume of solution, so it is directly proportional to the number of moles.
So, we can use a rule of three as follows:
aluminum ion --- aluminum sulfate
2 --- 1
x --- 0.646 M
So:
\(\begin{gathered} \frac{2}{x}=\frac{1}{0.646M} \\ 2\cdot0.646M=x\cdot1 \\ 1.292M=x \\ x=1.292M \end{gathered}\)So, the concentration of aluminum ion in this solution is 1.292 M.
Light from a laser pointer goes through a window composed of crown glass, which has an index of refraction of 1. 523. What is the speed of the light while it is in the window glass?.
Answers
Answer: 1.97 x 10^8 m/s
Explanation:
What is the job of white blood cells? How do they
behave?
Answers
Answer: they help stop bacteria and diseases
Explanation:
Answer:
thier job is to fight diseaeses, they make up the immune system of the body
Explanation:
PLS HELP What is the name of the value located in the top right of an element?
Answers
Answer:
It's the elements atomic mass and the number of neutrons and protons in an atom.
Explanation:
How much heat is evolved in the formation of 35.0 grams of Fe2O3(s) at 25°C and 1.00 atm pressure by the following reaction?4Fe(s) + 3O2(g) → 2Fe2O3(s)(kJ/mol) 0 0 −824.2a. 90.4 kJb. 180.7 kJc. 151 kJd. 360.1 kJe. 243. 9 kJ
Answers
We calculate the moles of Fe2O3 formed, then use the stoichiometry and given enthalpy of formation to calculate the heat evolved, which is approximately -361.6 kJ. The answer closest to this is option (d) 360.1 kJ.
We use the enthalpy of formation and stoichiometry of the reaction to determine the heat released during the creation of 35.0 g of Fe2O3. Prior to calculating the moles of O2 reacted, we first calculate the moles of Fe2O3 formed. The heat developed is then calculated using the reaction's specified enthalpy of formation. The enthalpy change and the formation of moles of Fe2O3 are the causes of the heat evolution. The closest response is option (d) 360.1 kJ since the enthalpy change is negative, suggesting an exothermic process. As a result, at 25 °C and 1.00 atm of pressure, the reaction produces 361.6 kJ of heat during the creation of 35.0 g of Fe2O3.
learn more about moles of Fe2O3 formed here:
https://brainly.com/question/21803958
#SPJ11
what happens to the rate of a reaction as the reaction progresses
Answers
What different about paratisim compared to the other biological relationships such as mutualism,predation, competition, and commensalism
A parasitic organism causes harm to other organisms as a result of the symbolic relationship
The parasite must utilize a host organism that may or may not die as a result of the relationship
The host is neither helped nor hurt by the relationship and interaction with the parasitic organism
Both the parasite and the host are helped by the relationship and interaction with the parasitic organism
Answers
Answer:
Mutualism is a symbiotic relationship in which both species benefit. Commensalism is a symbiotic relationship in which one species benefits while the other species is not affected. Parasitism is a symbiotic relationship in which one species (the parasite) benefits while the other species (the host) is harmed
Symbiosis is a close relationship between two species in which at least one species benefits.
Mutualism is a symbiotic relationship in which both species benefit.
Commensalism is a symbiotic relationship in which one species benefits while the other species is not affected.
Parasitism is a symbiotic relationship in which one species (the parasite) benefits while the other species (the host) is harmed.
Answer:
B..
Explanation: Internet
An acid is hydrogen and one or more nonmetals.
Select one:
True
False
Answers
Answer:
true
Explanation:
hope this helps!
brainliest please
have a great day
please fully rate
please no reporting. (it makes me sad)
An acid is hydrogen and one or more nonmetals. This statement is true. Therefore, option A is correct.
What is non metal ?A nonmetal is a chemical element that typically doesn't have a lot of metallic characteristics; examples include colourless vapors and glossy solids. When compared to metals, nonmetals' electrons exhibit different behaviour.
Natural substances known as non-metals are brittle and thermally and electrically inert (can not be easily rolling, moulding, extruding or pressing). The non-metallic elements in the periodic table are hydrogen, carbon, nitrogen, oxygen, phosphorus, arsenic, and selenium.
Non-metals are substances that lack luster, sonority, ductility, malleability, and are poor conductors of heat and electricity. They are soft and dull in appearance. Take oxygen, hydrogen, sulphur, etc. as examples.
Thus, option A is correct.
To learn more about the non metal, follow the link;
https://brainly.com/question/20602843
#SPJ2
What mitigation measures can communities do to reduce the damage and impact of sudden geologic hazards?
Answers
Explanation:
require an emergency support immediately
Based on this, which of the reactions below
are double-replacement reactions?
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
2 AgNO3(aq) + Zn(8) + 2Ag(s) + Zn(NO3)2(aq)
Pb(NO3)2(aq) + 2KI(aq) + 2KNO3(aq) + Pb12(s)
BaCl2(aq) + Na2SO4 (aq) + BaSO4(s) + 2NaCl(aq)
Answers
Answer:
BaCl2(aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl(aq)
Explanation:
Chemical reactions are of different types namely; combination reaction, displacement reaction, double displacement reaction etc. Double displacement/replacement reaction is that reaction in which an exchange of ions occur between two reacting ionic compounds to form new products.
In this chemical reaction/equation;
BaCl2(aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl(aq)
2Cl- and SO42- are the ions exchanged in this reaction. Barium (Ba) displaces SO42-, while Sodium (Na) displaces 2Cl-, hence it is called DOUBLE DISPLACEMENT OR REPLACEMENT because the displacement involves two compounds/ions.
Complete and balance the equation for the reaction of sodium with water.
Na+_H2O+ H2
Answers
2Na + 2H2O → 2NaOH + H2
The balanced equation
If the molar mass of Na2SO4•nH2O is 304.04 g/mol, what is the hydration number 'n'?A. 10B. 9C. 7D. 8
Answers
Chemistry => The Mole => Percent of water in a Hydrate
We have a compound that is hydrated with "n" moles of water. To determine the moles of water, what we will do is take as a base a mole of the dry compound, that is, 1 mol of Na2SO4.
We will determine the mass of one mol of the dry compound and the difference between the hydrated compound and the dry compound will be the water content.
Let's see what is the molar mass of the dry compound: Na2SO4
Element Mass
Na 2 x 22.99 =45.98 g/mol
S 1 x 32.065 = 32.065 g/mol
O 4 x 15.999 = 63.996 g/mol
Sum 142.04 g/mol
So, we have that one mol of Na2SO4 has a mass of 142.04.
Now, the water in 1 mol of the hydrated compound will be:
\(304.04-142.04=161.999g\)Now, we divide the mass obtained by the molar mass of water, 18.01 g/mol:
\(\begin{gathered} molH_2O=161.99gH_2O\times\frac{1molH_2O}{18.01gH_2O} \\ \\ molH_2O=9 \end{gathered}\)In the hydrated compound, we have 9 moles of H2O for each mol of Na2SO4.
Therefore the ANSWER will be B. 9
a. the vapor pressure of bromobenzene at 40°c is 10 mm hg. determine the phase of bromobenzene at 40°c, 1 atm. b. the normal boiling point of fluorobenzene is 84.7°c. what is the phase of fluorobenzene at 25°c, 1 atm?
Answers
A. The phase of bromobenzene at 40°C and 1 atm is a *liquid*.
b. The phase of fluorobenzene at 25°C and 1 atm is a *liquid*.
A- According to the given information, the vapor pressure of bromobenzene at 40°C is 10 mm Hg. Vapor pressure is the pressure exerted by the vapor of a substance in equilibrium with its liquid phase at a given temperature. When the vapor pressure of a substance equals the external pressure (1 atm in this case), the substance boils and transitions from the liquid phase to the gas phase.
Since the vapor pressure of bromobenzene at 40°C (10 mm Hg) is lower than the external pressure of 1 atm, bromobenzene does not reach its boiling point and remains in the liquid phase.
B- The normal boiling point of a substance is the temperature at which its vapor pressure equals the external pressure of 1 atm. In this case, the normal boiling point of fluorobenzene is given as 84.7°C.
Since the temperature of 25°C is below the normal boiling point of fluorobenzene, it does not reach its boiling point and remains in the liquid phase. At 25°C and 1 atm, the vapor pressure of fluorobenzene is lower than the external pressure, indicating that it will not undergo a phase change to the gas phase. Therefore, fluorobenzene exists as a liquid at 25°C and 1 atm.
learn more about Vapour pressure
https://brainly.com/question/33652329
#SPJ4
At what temperature will the following processes be spontaneous?
(a) ΔrH=−18kJ and ΔrS=−60J/K
(b) ΔrH=+18kJ and ΔrS+60J/K
(c) ΔrH=+18kJ and ΔrS=−60J/K
(d) ΔrH=−18kJ and ΔrS=+60J/K
Answers
At 300 K temperature, all processes will be spontaneous. Therefore, option 1 is correct.
Use the Gibbs free energy equation:
ΔG = ΔH - TΔS
where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
If ΔG is negative (ΔG < 0), the process is spontaneous at that temperature. If ΔG is positive (ΔG > 0), the process is non-spontaneous at that temperature. And if ΔG is zero (ΔG = 0), the process is at equilibrium.
a) dH < T×dS
-18000 < T×(-60)
T < 300
The temperature must be lower than 300 K
b) dG = H- T×dS < 0
18000 < T×60
300 < T
The temperature must be higher than 300 K
(c) 18 kJ + 60 J/K × T < 0
60 J/K × T < -18 kJ
T < (-18 kJ / 60 J/K)
T < -300 K (not physically meaningful since the temperature cannot be negative)
(d) -18 kJ + 60 J/K × T < 0
60 J/K × T > 18 kJ
T > 300 K
To learn more about the spontaneous process, follow the link:
https://brainly.com/question/12319501
#SPJ12
Your question is incomplete, most probably your question was:
At what temperature will the following processes be spontaneous?
(a) ΔrH=−18kJ and ΔrS=−60J/K
(b) ΔrH=+18kJ and ΔrS+60J/K
(c) ΔrH=+18kJ and ΔrS=−60J/K
(d) ΔrH=−18kJ and ΔrS=+60J/K Options are:
1. 300K,300K,negative,300K
2. 200K,negative, 300K, 300K
3. 300K,300K,300K, negative
4. negative,300K,300K,300K,
2. How many moles are in 7.30 X 10^23 molecules of NaCl?
Answers
Answer:
\( \huge{ \boxed{1.213 \: \text{moles}}}\)
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
\( \bold{n = \frac{N}{L}} \\ \)
where
n is the number of molesN is the number of entitiesL is the Avogadro's constant which is 6.02 × 10²³ entitiesFrom the question
N = 7.30 × 10²³ NaCl molecules
\(n = \frac{7.30 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{7.30}{6.02} \\ = 1.2126\)
We have the final answer as
1.213 molesWhich of the following is an example of a molecule with different atoms?
Question 6 options:
hydrogen - H2
water - H2O
oxygen - O2
helium - He
Answers
Answer:
Oxygen -02 Because it present oxygen
a normal penny has a mass of about 2.5g. if we assume the penny to be pure copper (which means the penny is very old since newer pennies are a mixture of copper and zinc), how many atoms of copper do 9 pennies contain?
Answers
9 pennies contain approximately \(2.13 x 10^23\) atoms of copper.
To solve this problem, we need to use the following steps:
Determine the molar mass of copper.
Convert the mass of 9 pennies from grams to moles.
Use Avogadro's number to calculate the number of atoms of copper.
Step 1: The molar mass of copper (Cu) is approximately 63.55 g/mol.
Step 2: The mass of 9 pennies is:
9 pennies x 2.5 g/penny = 22.5 g
Converting this mass to moles, we get:
22.5 g / 63.55 g/mol = 0.354 moles
Step 3: Using Avogadro's number (\(6.022 x 10^23 atoms/mol)\), we can calculate the number of atoms of copper:
Therefore, 9 pennies contain approximately\(2.13 x 10^23 a\)toms of copper.
Learn more about molar mass
https://brainly.com/question/22997914
#SPJ4
differentiate between a promoter and an inhibitor
Answers
Promoters are substances that increase the catalytic activity, even though they are not catalysts by themselves.
Inhibitors are sometimes referred to as "negative catalysts" since they decrease the reaction rate.
How many sigma and how many pi bonds are in the molecule shown below?
Answers
Correct op tion is B, sigma C−C bonds =3, sigma C−H bonds =2+1+3=6 , pi C−C bonds =1+1=2
What are sigma and Pi bonds?The overlapping of atomic orbitals distinguishes sigma and pi bonds from other varieties of covalent connections. Atomic orbitals colliding together create covalent bonds. Sigma bonds are created when two atomic orbitals overlap one another head-to-head, whereas pi bonds are created when two atomic orbitals overlap one another laterally. The coaxial overlapping of the two orbitals with identical energies results in the formation of sigma bonds (). The single bonds are typically sigma bonds. The lateral overlapping of two orbitals with similar energies results in the formation of pi bonds (). One sigma bond and one pi bond are found in a double bond, while two sigma bonds and two pi bonds are found in a triple bond. Sigma bonds have orbitals that are aligned with the internuclear axis, as opposed to pi bonds, which have orbitals that are perpendicular to the internuclear axis. As a result, sigma bonds have greater effective orbital overlap. The sigma bond is stronger than the pi bond because to the large difference in orbital overlap.
To know more about the sigma bond, visit:
https://brainly.com/question/14018074
#SPJ1
Wy do rough, uneven pebbles become smooth and rounded
Answers
Answer:
Due to erosion
Explanation:
When a pebble breaks off from a greater piece it is rough. After being weathered down and constantly hit against by other masses, the side will wear down and smoothen out
what is the percent composition of salicylic acid?
Answers
The percent composition of salicylic acid is C7H6O3, or 60.87%C, 4.4%H, and 34.75%O
if the molarity of an acid is 5.5, what is the OH
Answers
When the molarity of an acid is 5.5, the concentration of hydroxide ions (OH-) in the solution is approximately 1.82 * 10^{-15} mol/L.
To determine the concentration of hydroxide ions (OH-) in a solution when the molarity of an acid is 5.5, we need to use the concept of "molarity" and understand the relationship between an "acid" and its corresponding base.
Step 1: Determine the concentration of hydronium ions (H3O+)
Molarity of an acid represents the concentration of hydronium ions (H3O+) in the solution. In this case, the molarity of the acid is 5.5, which means the concentration of H3O+ ions is 5.5 mol/L.
Step 2: Use the ion product constant of water (Kw)
In any aqueous solution, there is a relationship between the concentration of hydronium ions (H3O+) and hydroxide ions (OH-). This relationship is described by the ion product constant of water (Kw), which is 1.0 *10^{-14}at 25°C.
Kw = [H3O+] [OH-]
Step 3: Calculate the concentration of hydroxide ions (OH-)
Now, we can use the Kw value and the concentration of H3O+ ions to find the concentration of OH- ions in the solution.
1.0 *10^{-14} = (5.5) [OH-]
Divide both sides of the equation by 5.5:
[OH-] =\frac{( 1.0 *10^{-14}) }{ 5.5}
Step 4: Solve for [OH-]
[OH-] ≈ 1.82 * 10^{-15} mol/L
So, when the molarity of an acid is 5.5, the concentration of hydroxide ions (OH-) in the solution is approximately 1.82 * 10^{-15} L.
learn more about hydroxide ions Refer: https://brainly.com/question/14576028
#SPJ11
can there be an ionic bonding between 2 metals or 2 non-metals?
Answers
Answer:
No
Explanation:
Ionic bonding is where an one atom wants to gain e- while the other wants to lose e-
Such as NaCl.
for the reaction, Pb(no3)2 + 2kI yields pbi 2 + 2 kno3, how many moles of lead iodine are produced from 300 g of potassium iodine