George Tomlin, RMA, has been working for several years in a specialty practice. He applies for a position closer to his home with better hours and more pay. This office, however, sees patients with a variety of illnesses. For the first time since he graduated from college, he is encountering words and procedures with which he is not familiar. What is the best way for George to review his basic medical terminology? What should George do when he encounters a new word? What are some good ways for him to learn the new vocabulary for his new position?"
Answers
Answer:
George Tomlin can make some flashcards, this method really works because the human brain learns by visual cues, looking at the word to recall the definition can help train your brain for memorization.He can also record himself, simply speaking and hearing medical terminology out load can also help you learn. One simple trick is to record yourself saying these medical terms and their definitions. The act of recording them will create aural flashcards. Listening to your recording will help you remember the words when you come across them in your reading. Another good tip would be memorizing the root words. Medical terminology is based on Latin and Greek root words. Understanding the word parts will help you understand complex medical terms. Often knowing part of a word will help you figure out the meaning of the entire word. For instance, knowing the “branch” is the root for terms related to the respiratory system, makes it easier to understand that “bronchial” or “bronchitis” is also related to the lungs or breathing.
Explanation:
For George Tomlin, the best way to review the basic medical terminologies and methods is by visual as well as by auditory ways.
George can read the words carefully by going through them in books or from online resources.He can listen carefully the words in online lectures and tutorials and understand the meaning of the terminologies.When George encounters a new word :
he can look up for the meaning of the word in the medical textbooks or from any online sources.he can understand the meaning by discussing with his colleagues.the new words has its roots, abbreviations, suffixes and prefixes in a close proximity which makes the terminology easy and also provides quick reference.Some of the best ways for George to learn the new vocabulary for the new position are :
George can breakdown the words in such a way that helps him to memorize the terminology less and understand more.Learn more in details about the origin and structure of the medical terms and discuss more often with his colleagues.Learn More :
https://brainly.com/question/15010232

Related Questions
what is the mass, in grams, of 4.00 × 1022 helium atoms?
Answers
The mass of 4.00 × 10^22 helium atoms is approximately 0.265 grams.
To determine the mass of 4.00 × 10^22 helium atoms, we need to know the molar mass of helium, which is 4.0026 g/mol. The molar mass represents the mass of one mole of helium atoms.
To calculate the mass of a given number of helium atoms, we can use the following steps:
Convert the given number of helium atoms to moles:
Moles = Number of atoms / Avogadro's number
Moles = 4.00 × 10^22 atoms / 6.022 × 10^23 atoms/mol
Calculate the mass using the molar mass of helium:
Mass = Moles × Molar mass
Mass = Moles × 4.0026 g/mol
Now, substituting the values:
Moles = (4.00 × 10^22 atoms) / (6.022 × 10^23 atoms/mol) ≈ 0.0663 mol
Mass = (0.0663 mol) × (4.0026 g/mol) ≈ 0.265 g
Therefore, the mass of 4.00 × 10^22 helium atoms is approximately 0.265 grams.
Know more about Helium here:
https://brainly.com/question/5596460
#SPJ11
Of the following elements ____ can form a rare +4 ion *
a) Aluminum
b) Lead
c) Krypton
d) Uranium
Answers
Answer:
d is the answer.........
How many valence electrons does magnesium(Mg) have?
Answers
Magnesium have two valence electrons. Because the outer energy level for the magnesium atom is 3 and it has two electron in this energy level.
The Valence electrons are defined as the electrons that located in the outermost electron shell of an atom. These valence electrons being the furthest from the nucleus and thus the least tightly held by the atom are the electrons that participate in bonds and reactions. The number of valence electrons that an element has determines its reactivity, electronegativity and the number of bonds it can form. We can use the periodic table to help to determine how many valence electrons an element specifically a neutral atom of the element has. Looking at the group that the element is in as the group number indicates the number of valence electrons that the element has.
To learn more about Valence electron please visit:
https://brainly.com/question/371590
#SPJ4
Smoking till my eyes roll back like the omen lyrics.
Answers
All objects made of matter create what pull?
Answers
Answer:
energy
that seems like the most relevant answer
CsH16 +12028CO2 +8H₂O
What is the ratio of octene (C8H16) to
oxygen in the reaction?
Answers
The ratio of octene to oxygen is 1:12.
To determine the ratio of octene (C8H16) to oxygen (O2) in the given reaction, we need to examine the balanced chemical equation. However, the equation you provided does not seem to be balanced. The coefficients for each compound must be determined to achieve a balanced equation before we can calculate the desired ratio.
Assuming you meant the combustion reaction of octene, a balanced equation would be:
C8H16 + 12O2 → 8CO2 + 8H2O
From the balanced equation, we can see that for every 1 mole of octene (C8H16), we require 12 moles of oxygen (O2) to completely react.
This means that for every 1 mole of octene, we need 12 moles of oxygen to fully combust the octene and produce the corresponding amounts of carbon dioxide (CO2) and water (H2O) as shown in the balanced equation.
For such more questions on combustion
https://brainly.com/question/13251946
#SPJ8
4. A molecule is made up of at least _____________________________________ different atoms.
Answers
Answer:
Two similar atoms or different atoms
Explanation:
A molecule is made up of at least two similar atoms or different atoms.
A molecule is the smallest particle of a substance capable of independent existence.
Monoatomic molecule is made up of one atomDiatomic molecule is made up of two atoms bounded together. Polyatomic molecule are made up of more than two molecules bounded together.Answer: Two similar atoms or different atoms.
Have a great day!
Which environmental impact is associated with the use of nuclear power plants?.
Answers
Answer:
A major environmental concern related to nuclear power is the creation of radioactive wastes such as uranium mill tailings, spent (used) reactor fuel, and other radioactive wastes. These materials can remain radioactive and dangerous to human health for thousands of years.
If you can answer this you a OG
Calculate the new volume if 12.78 L of a gas at -50*C is heated to a temperature of 28*C
Answers
When the gas is heated from -50°C to 28°C, the new volume is approximately 17.24 L.
To calculate the new volume of a gas when it is heated from -50°C to 28°C, we can use the combined gas law, which relates the initial and final conditions of temperature and volume.
The combined gas law is expressed as:
(P1 * V1) / T1 = (P2 * V2) / T2
Where:
P1 = Initial pressure
V1 = Initial volume
T1 = Initial temperature in Kelvin
P2 = Final pressure (assumed constant in this case)
V2 = Final volume (to be calculated)
T2 = Final temperature in Kelvin
First, we need to convert the initial and final temperatures from Celsius to Kelvin:
Initial temperature in Kelvin (T1) = -50°C + 273.15 = 223.15 K
Final temperature in Kelvin (T2) = 28°C + 273.15 = 301.15 K
Since the pressure is assumed to be constant, we can simplify the equation:
(V1 / T1) = (V2 / T2)
Now we can substitute the given values into the equation:
(12.78 L / 223.15 K) = (V2 / 301.15 K)
Cross-multiplying and solving for V2:
V2 = (12.78 L * 301.15 K) / 223.15 K
V2 ≈ 17.24 L
For more such question on volume. visit :
https://brainly.com/question/1972490
#SPJ8
2. Which of the following shows the correct, balanced equation for the reaction shown below?
CH4+0₂H₂O + CO₂
O CH, + O, → CHO
O CH4 +20₂ → 2 H₂O + CO₂ OCH, + O2 → H2O +CO,
OCH4 +0₂ → CH₂0
Answers
The balanced equation is \(\text{CH}_{4}+2\text{O}_{2} \longrightarrow \text{CO}_{2}+2\text{H}_{2}\text{O}\).
The correct balanced chemical equation is CH₄ + 2 O₂\(\rightarrow\) CO₂ + 2 H₂O.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/29130807
#SPJ5
Methanol and ethanol are said to be miscible in one another. What does this tell you?
Answers
Answer:
That they are miscible in one another
in each reaction box, select the best reagent and conditions from each list.
Answers
The best reagent and conditions from each list :
1) AlCl₃ - CH₃Cl at room temperature
2) HNO₃ -H₂SO₄ at room temperature
3) Fuming HNO₃ -H₂SO₄ at 90-100 ⁰C heat .
Benzene will be converted to the toluene by the Friedel Craft Alkylation of the benzene . In this reaction the reagent AlCl₃ and the CH₃Cl is used .
Dinitritoluene will be prepared from the toluene by the Nitration . The reaction is uses the Electrophilic substitution mechanism . The reagents that used are HNO₃ and the H₂SO₄ at room temperature.
This reaction is the extended nitration of the toluene . The Further nitration is done in the extreme condition . The temperature of the reaction is increased to the 90- 100 ⁰ C.
To learn more about reagents here
https://brainly.com/question/28504619
#SPJ4

in an experiment a piece of magnesium ribbon was cleaned with steel wool 2.4g of the clean ribbon was placed in a crucible and completely... In an experiment a piece of magnesium ribbon was cleaned with steel wool 2.4g of the clean ribbon was placed in a crucible and completely burnt in oxygen.after cooling the product weighted 4.0g. (a)explain why it was necessary to clean the magnesium ribbon? (b)what observation was made in the crucible after burning? (c)why was there an increase in mass? (d)write the equation for reaction which took place in the crucible? (e)the product in the crucible was shaken with water and filtered.
Answers
(a) It was necessary to clean the magnesium ribbon in order to remove any impurities or contaminants that might be present on the surface of the ribbon. These impurities could interfere with the burning process or alter the final products of the reaction.
(b) After burning, it is likely that an ash or residue was observed in the crucible.
(c) The increase in mass may be due to the production of new products during the burning process. These products may include magnesium oxide, which is a white solid, or other compounds formed through the reaction of magnesium with oxygen.
(d) The equation for the reaction that took place in the crucible is:
2Mg + O2 -> 2MgO
(e) When the product in the crucible is shaken with water, it is likely that any soluble products, such as magnesium hydroxide, will dissolve in the water. The remaining solid material can then be filtered out, leaving a solution containing the dissolved products.
The magnesium ribbon was cleaned to remove any impurities that could affect the reaction. After burning magnesium in oxygen, the resulting product is magnesium oxide, and the mass increases because the magnesium combines with the oxygen. The reaction equation is 2Mg + O2 -> 2MgO.
Explanation:(a) It was necessary to clean the magnesium ribbon with steel wool to remove any surface oxidation or impurities that could affect the reaction.
(b) After burning, an observation that could be made in the crucible would be a white ash-like substance, which is magnesium oxide.
(c) There was an increase in mass because when magnesium reacts with oxygen, it gains mass as it combines with the oxygen to form magnesium oxide.
(d) The equation for the reaction is: 2Mg + O2 -> 2MgO
(e) When the product in the crucible was shaken with water and filtered, this procedure would typically be used to isolate the product from any remaining unreacted magnesium or other reaction by-products.
https://brainly.com/question/31503589
#SPJ2
Please Help. Thank You!

Answers
2. What’s different inside of an atom to make them into different elements is that the number of protons in an atom is the defining feature of an atom. This is what makes a element different from another.
Hope this helps!!
what is the temperature of the liquid at vaporization (boiling/condensing)?
Answers
Draw the stracture of 2-bromo-4-chloro-3, 3-dimethylhex-1-ene
Answers
answer :
this is the structure if you want it


Chemical treatment (of hazardous waste) fefers to the treatment methods that are used io elfect the complete brealutovin of hazardour wase in
Answers
Chemical treatment of hazardous waste refers to the treatment methods that are used to effect the complete breakdown of hazardous waste. The hazardous waste is chemically processed in chemical treatment to make it less harmful and less toxic.
There are several different chemical treatment methods used to treat hazardous waste, including oxidation, reduction, neutralization, and precipitation. In oxidation, the waste is treated with oxidizing agents to convert the waste into a less harmful form. In reduction, the waste is treated with reducing agents to convert the waste into a less harmful form. In neutralization, an acid or base is added to the waste to neutralize its pH.
In precipitation, a chemical is added to the waste to cause it to precipitate out of solution. Chemical treatment is one of several methods used to manage and dispose of hazardous waste in an environmentally responsible manner.
To know more about Chemical treatment visit:
https://brainly.com/question/14690736
#SPJ11
A configuração eletrônica de um átomo neutro no estado fundamental é 1s2 2s2 2p6 3s2 3p5. O número de orbitais vazias remanescente no nível principal M é: a)0 b)1 c)5 d)6 e)10
Answers
Given configuration
\(\\ \bull\leadsto 1s^22s^22p^63s^23p^5\)
Its a halogen family element hence valency is 1The element present in 3rd period Group 17The element is Bromine(Br)alcoholic fermentaion occurs when there is a lack of what
Answers
Which type of fossil can be described as dead wood that has turned into stone?
a. cast fossil
b. sedimentary fossil
c. petrified
d. mold fossil
Answers
Answer:
The answer is c hope it is right if it is rong please tell me tho :)
how to tell if something is more soluble in solubility curve
Answers
Answer:
To find the least soluble substance at a given temperature we follow the temperature line up and the first substance curve we hit is the least soluble. For most soluble it is the same procedure except the last substance curve hit is the most soluble.
To determine if a substance is more soluble or less soluble based on a solubility curve, you need to compare the solubility values at different temperatures. Here's how you can interpret a solubility curve:
1. Higher Points on the Curve: If a point on the curve is higher, it indicates that the substance is more soluble at that temperature. In other words, at higher temperatures, the substance can dissolve in a greater amount.
2. Lower Points on the Curve: If a point on the curve is lower, it means that the substance is less soluble at that temperature. In this case, at lower temperatures, the substance can dissolve in a smaller amount.
3. Comparing Points: By comparing the solubility values at different temperatures, you can determine which temperature has a higher solubility and which has a lower solubility. The steeper the slope of the curve, the faster the increase or decrease in solubility with temperature.
Solubility curves provide a graphical representation of the relationship between temperature and solubility. They allow you to determine the solubility characteristics of a substance and how it changes with temperature.
To learn more about solubility curves, visit:
brainly.com/question/14366471
#SPJ11
The equations are not balanced. Which equation would have the same coefficients in the same order as 2CO2 + 3H20 C2H6O + 3O2?
Answers
Answer:
Fe + H2SO4 Fe2(SO4)3 + H2
Explanation:
its the answer on my answer key
Answer:
C- Fe + H2SO4 Fe2(SO4)3 + H2
Explanation:
The person above me is right. I also just took the test.
i’m too dumb for school.
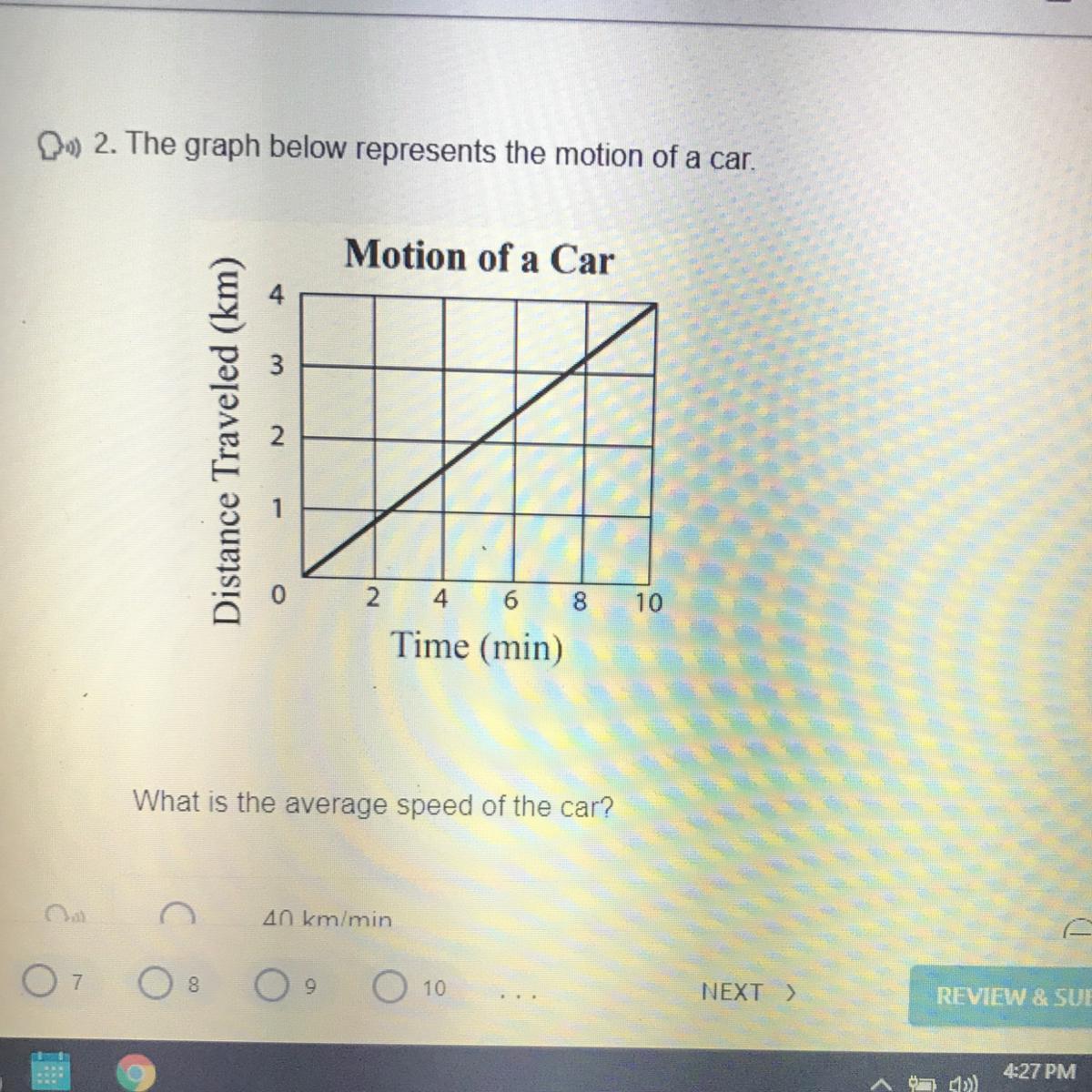
Answers
Answer:
every two minutes the car moves 1km
Explanation:
what does the t cell do to your body?
Answers
Answer: it's part of the immune system that focuses on specific foreign particles. Rather than generically attack any antigens, T cells circulate until they encounter their specific antigen. As such, T cells play a critical part in immunity to foreign substances !
Explanation:
How many moles of SiO2 are in 15.5g
Answers
The number of moles in 15.5g of silicon dioxide is 0.258moles.
How to calculate number of moles?The number of moles in a substance can be calculated by dividing the mass of the substance by its molar mass as follows:
moles = mass ÷ molar mass
Moles is the amount of substance of a system which contains exactly 6.02214076 × 10²³ elementary entities.
Molar mass of silicon dioxide is 60.08 g/mol
moles = 15.5g ÷ 60.08g/mol
moles = 0.258moles
Therefore, 0.258moles is the amount of moles in 15.5g of silicon dioxide.
Learn more about moles at: https://brainly.com/question/12513822
#SPJ1
you are asked to help with the radiodating of a plant based sample excavated from an archaeological site. the rate of 14c decay in the sample is 81.0% of the 14c decay rate in a living plant. what is the estimated age of the archaeological sample? (the half-life for 14c is 5730 years.)
Answers
The estimated age of the archaeological plant-based sample is approximately 1912 years.
1. Understand that decay refers to the process where the radioactive isotopes, such as 14C, break down over time, and half-life refers to the time it takes for half of the radioactive isotopes to decay.
2. Given that the 14C decay rate in the archaeological sample is 81.0% of the decay rate in a living plant, this means that the sample has 0.81 times the amount of 14C as a living plant.
3. Since the half-life of 14C is 5730 years, you can use the decay formula to calculate the age of the sample:
N = N0 * (1/2)^(t / half-life)
where N is the final amount of 14C, N0 is the initial amount, t is the age of the sample, and half-life is the half-life of 14C (5730 years).
4. Plug in the values given:
0.81 = 1 * (1/2)^(t / 5730)
5. Solve for t:
t / 5730 = log(0.81) / log(0.5)
t = 5730 * (log(0.81) / log(0.5))
t ≈ 1912 years
Learn more about half-life : https://brainly.com/question/11152793
#SPJ11
The cells of a tomato contain mostly an aqueous solution of sugar and other substances. If a typical tomato freezes at -2.5 °C, what is the molality of the sugar in solution? (Hint: sugar is a covalent compound, it does not dissociate in water)
Answers
Answer:
1.35 m
Explanation:
We can solve this problem by using the freezing point depression formula:
ΔT = Kf * m * iWhere:
ΔT is the temperature difference between the freezing point of the pure solvent (water) and the solution. In this case it is (0 °C - -2.5 °C = 2.5 °C).Kf is the cryoscopic constant, for water it is 1.853 °C*kg/mol.m is the molality.i is the van't Hoff factor, as sugar does not dissociate in water, it has a value of 1.We input the data:
2.5 °C = 1.853 °C*kg/mol * m * 1And solve for m:
m = 1.35 mSelect the correct arrows. Which arrows indicate weathering and erosion? sedimentary rocks metamorphic rocks igneous rocks

Answers
Answer:
igneous to sedimentary rock
Explanation:
Answer:
ingeous rocks to sedimentary rocks
Scientific knowledge can withstand the test of time because
A.
scientific theories have been proven beyond doubt.
B.
it is open to change as new evidence or data is discovered.
C.
advancements in technology have no impact on science.
D.
exceptions can be made regarding scientific laws.
Answers
B
Hope that helped
Answer: Its B for the Study Island assignment
Explanation:
What is the chemical equation C+O-CO2
Answers
Answer:
arbon monoxide reacts with oxygen to produce carbon dioxide. Write the balanced chemical equation for this reaction.
...
Example.
Step Result Equation balanced?
1 carbon monoxide + oxygen → carbon dioxide
2 CO + O 2 → CO 2
3 Reactants: 1 × C, (1 × O) + (2 × O) = 3 × O. Products: 1 × C, 2 × O Not balanced.
4 2CO + O 2 → CO