for the following equilibrium, nico3(s)↽−−⇀ni2 (aq) co2−3(aq) the addition of which of the following substances would cause the equilibrium to shift to the left?
Answers
The addition of Ni2+ ions or CO32- ions will cause the equilibrium to shift to the left for the following equilibrium: NiCO3 (s) ⇌ Ni2+ (aq) + CO32- (aq).
In the given equilibrium, NiCO3 (s) ⇌ Ni2+ (aq) + CO32- (aq), nickel (II) carbonate is dissolved in water to form nickel (II) ions and carbonate ions. This is an example of a dissociation reaction that occurs in equilibrium. The forward reaction moves to the right, whereas the reverse reaction moves to the left.In order to determine which substance will cause the equilibrium to shift to the left, we need to recall Le Chatelier's principle.
According to Le Chatelier's principle, a system at equilibrium will respond to any external stress in a way that minimizes the stress.In this case, if we add more Ni2+ ions or CO32- ions to the system, the equilibrium will shift to the left in order to minimize the stress. This is because adding more Ni2+ ions or CO32- ions will increase the concentration of the products, which will cause the reverse reaction to proceed to form more reactants.
To know more about equilibrium visit:
https://brainly.com/question/30694482
#SPJ11
Related Questions
A sample of gas has a volume of 2.00L at 25.0 degrees Celcius and 1.08atm. What volume ( in liters) will it have at 100 degrees Celcius and 1.5atm?
Answers
Answer:
Explanation:
The question requires us to calculate the volume (in L) of a gas under the conditions given.
The following information was provided by the question:
Initial volume of gas = V1 = 2.00 L
Initial temperature of gas = T1 = 25.0 °C
Initial pressure of gas = P1 = 1.08 atm
Final temperature of gas = T2 = 100 °C
Final pressure of gas = P2 = 1.50 atm
To solve this problem, we'll need to apply the equation of ideal gases (shown below) twice: first, to determine the number of moles of gas, and again to calculate the volume of gas under the final conditions. Note that we'll determine the number of moles of gas under the initial conditions because this amount won't change (as we're talking about the same sample of gas).
\(undefined\)What is "the cloud"?
A. The delivery of services and storage of data on multiple servers
using the Internet
B. A computer that provides services to and stores data but cannot
be accessed by other computers
O c. A system of devices that can communicate with one another
through analog signals sent through wires
D. A way to send analog data from one specific computer to another
computer that stores it as digital data
Answers
A
Explanation:
no proof just to know I'm internet gal
All samples of a specific substanice have the same chemical
composition
TRUE
FALSE

Answers
Answer:
true
Explanation:
When the ph of a solution decreases from 4. 0 to 2. 0, how does the concentration of h3o+ change?.
Answers
Hydronium ion concentration increases by 100 fold on decreasing the pH of solution from 4 to 2.
In order to compute pH, the log of the hydronium ion concentration was used.
pH = -log [Hydronium ion concentration]
So, at pH 2.0 number of hydronium ions are 10^-2
And at pH 4.0 number of hydronium ions are 10^-4
By decreasing pH there will be 100 times more hydronium ions.
To know more about hydronium ions, click here
brainly.com/question/12047300
#SPJ4
complete the following sentences to compare the following aqueous solutions: 0.5 m sucrose ( c12h22o11 ) and 0.35 m nano3 ,.
Answers
0.5 m sucrose solution is more concentrated than a 0.35 m NaNO3 solution. It is also more soluble, denser, and neutral in pH.
Aqueous solutions of sucrose (C12H22O11) and NaNO3 are both common solutions found in many everyday settings.
In order to compare the two, the following characteristics must be taken into consideration: molarity, solubility, density, and pH.
The molarity of a solution is the number of moles of solute per liter of solution. A 0.5 m sucrose solution has 0.5 moles of sucrose per liter, while a 0.35 m NaNO3 solution has 0.35 moles of NaNO3 per liter.
Thus, the sucrose solution is more concentrated than the NaNO3 solution.
The solubility of a substance is its ability to dissolve in a given solvent. Sucrose is highly soluble in water, while NaNO3 is only slightly soluble.
This means that the sucrose solution will dissolve faster and more completely than the NaNO3 solution.
The density of a solution is its mass per unit volume. Sucrose is denser than NaNO3, so a 0.5 m sucrose solution will be denser than a 0.35 m NaNO3 solution.
The pH of a solution is a measure of its acidity or alkalinity. Sucrose is neither acidic nor alkaline, while NaNO3 is slightly acidic.
Therefore, the sucrose solution will be neutral in pH, while the NaNO3 solution will have a slightly acidic pH.
In conclusion, a 0.5 m sucrose solution is more concentrated than a 0.35 m NaNO3 solution. It is also more soluble, denser, and neutral in pH.
to know more about sucrose refer here:
https://brainly.com/question/28869238#
#SPJ11
What type of change occurs when a counterfeit pen ink comes into contact with counterfeit money, and why does this reaction not occur when the counterfeit pen is used on genuine money printed in the United States
Answers
In conclusion, when counterfeit pen ink comes into contact with counterfeit money, the ink reacts and marks the paper with a particular color, which indicates whether the money is genuine or fake. In the United States, the ink used to print currency and the paper used to print it contain a special chemical that makes it possible for the pen to mark the surface but not to produce a chemical reaction.
When a counterfeit pen ink comes into contact with counterfeit money, the ink reacts and marks the paper with a particular color, which indicates whether the money is genuine or fake. This ink is utilized as a measure against counterfeit money, as it has a chemical reaction to specific chemicals that are found in fake banknotes.
What is the type of change that occurs when a counterfeit pen ink comes into contact with counterfeit money?
When counterfeit pen ink comes into contact with counterfeit money, the ink produces a chemical reaction that leads to a modification in the appearance of the ink. The color of the ink transforms to reveal whether or not the money is genuine. In most circumstances, the ink turns black if the money is not genuine and yellow or pale if the money is genuine.
Why doesn't this reaction occur when the counterfeit pen is used on genuine money printed in the United States?
In the United States, the ink used to print currency and the paper used to print it contain a special chemical that makes it possible for the pen to mark the surface, but not to produce a chemical reaction. This ink is intended to prevent individuals from being able to use a counterfeit pen to determine whether or not a banknote is real because the United States Secret Service, the organization in charge of counterfeit prevention, can track counterfeit banknotes with this technology.
to know more about counterfeit prevention visit:
https://brainly.com/question/30088488
#SPJ11
clouds are colloids. what are the dispersed particles
Answers
Answer:
Water droplets slowly forming until they reach a size that can no longer be suspended in the air.
Explanation:
When air becomes saturated with whater, it can no longer maintain water as a gas. Some water molecule come together and stay that way. They, in turn, coallesce with other nearby particles. This continues until the droplets are large enough that they begin to noticeably refract the sunlight, producting the visual effect we call clouds. The droplets are suspended in air - an heterogenous suspension called a colloid. At some point the colloid cloud begins to shed the larger water droplets, usually directly over my spot on the trail.
The decomposition of 65.9 g ammonium nitrate yields how many liters of dinitrogen monoxide at 2.65 atm and 303 k?
(N= 14.01 g/mol, H= 1.008 g/mol, O=16.00 g/mol)
NH4NO3(s) -> N2O(g)+2H2O(g)
Hint: R = 0.0821 L atm/(mol K)
Answers
It’s 7.72 it took a while but I found the answer
I need help finding the dimensional analysis
Answers
Dimensional analysis is a mathematical technique that involves analyzing the dimensions of physical quantities in order to determine the relationship between them.
It involves looking at the units of measurement for each quantity and using them to establish the relationship between them. This is an important technique in many fields of science, including physics, chemistry, and engineering. To perform dimensional analysis, you must first identify the physical quantities involved in the problem. Then, you must determine the units of measurement for each quantity. Once you have done this, you can use the principles of dimensional analysis to establish the relationship between the quantities. For example, let's say you are trying to find the relationship between the velocity of an object and its acceleration. You would first identify the physical quantities involved - velocity and acceleration. You would then determine the units of measurement for each quantity - velocity is typically measured in meters per second (m/s), while acceleration is typically measured in meters per second squared (m/s²).
Using the principles of dimensional analysis, you could then establish the relationship between velocity and acceleration by dividing the units of measurement for velocity by the units of measurement for acceleration. This would give you the dimensions of the relationship between the two quantities - in this case, meters per second divided by meters per second squared, or simply 1/second. In summary, dimensional analysis is a useful technique for analyzing the relationship between physical quantities. By understanding the dimensions of each quantity, you can establish relationships and solve problems more effectively.
Learn more about acceleration here :
https://brainly.com/question/12550364
#SPJ11
0.357 moles of sulfur dioxide to grams
Answers
2. How many moles are in 6.55 x 108 grams of H2O?
3.63x10^7 moles
Answers
Answer:
Who helped Hernán Cortés and his soldiers defeat the Aztec?
Which of the following acids has the strongest conjugate base?
Question options:
HClO
HClO4
HClO2
HClO3
HCl
Answers
Among the given options, the acid with the strongest conjugate base is HClO4 (perchloric acid).
The strength of a conjugate base depends on the stability of the resulting anion after the acid donates a proton. A stronger acid will have a weaker conjugate base.
In this case, HClO4 (perchloric acid) is the strongest acid among the options. When it donates a proton, the resulting conjugate base is ClO4- (perchlorate ion). The perchlorate ion is highly stable and delocalizes the negative charge across the four oxygen atoms, making it a very weak base.
On the other hand, the other acids (HCl, HClO2, HClO3) will have relatively weaker conjugate bases compared to HClO4.
Therefore, among the given options, HClO4 (perchloric acid) has the strongest conjugate base.
Learn more about conjugate bases: brainly.com/question/30086613
#SPJ11
Calculate the densities of the objects using the volumes found by displacement. Record your data
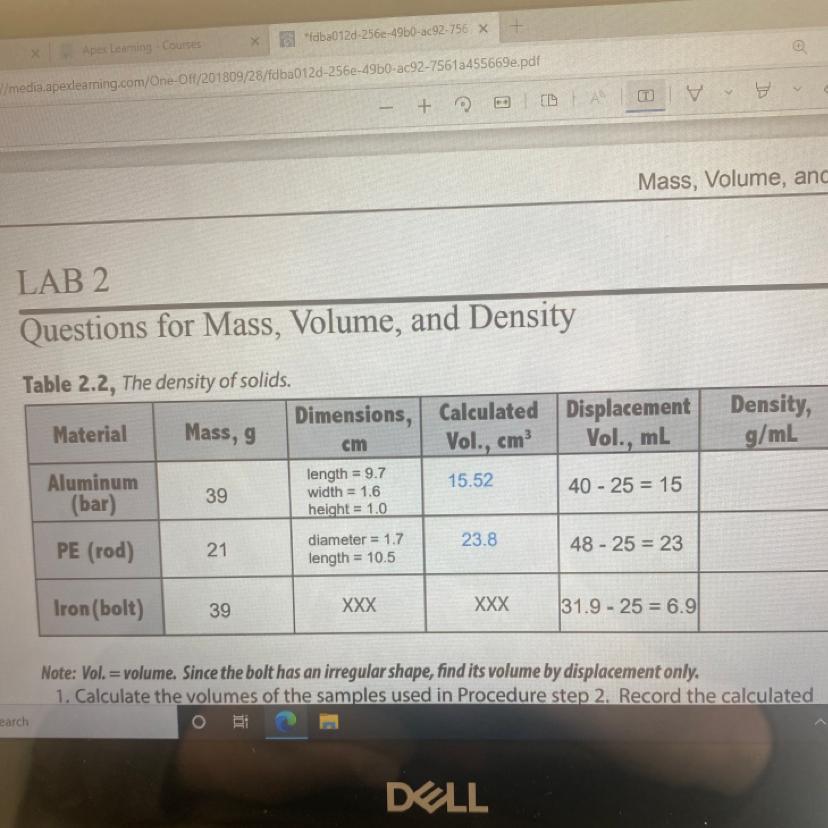
Answers
The densities of the substances are;
Aluminum bar = 2.6 g/ml
PE rod = 0.91 g/mL
Iron bolt = 5.65 g/mL
What is density?The term density is defined as the ratio of the mass to the volume of the object. We know that mass is an intrinsic property. This means that the density of the object can be used to identify what the object that is under study is. Let us now try to find the density of each of the objects.
In this case, we have the mass and the volumes of the objects as they have been shown in the table that have here. We can now be able to find the volume of each of the objects.
Density of aluminum bar = 39 g/ 15 g/mL = 2.6 g/ml
Density of the PE rod = 21 g/23 mL = 0.91 g/mL
Density of iron bolt = 39 g/6.9 mL = 5.65 g/mL
Learn more about density:https://brainly.com/question/15164682
#SPJ1
Calculate the atomic mass of Carbon if the two common isotopes of carbon have masses of 12.000 amu (98.89 % abundance) and 13.003 amu (1.11 % abundance).
Answers
Answer:
Average atomic mass =
(mass of isotope)(%abundance)+(mass of isotope) ( abundance ) / 100
avg. atomic mass =(12)(98.89) + (13)(1.11) /100
=1186.68 + 14.43 / 100
=1201.11 / 100
=12.0111
Đốt cháy hoàn toàn 0,1 mol axit đơn chức cần V lít O2 ở đktc. Thu được 0,3 mol CO2 và 0,2 mol h20 vậy giá trị V là
Answers
Answer:
Complete combustion of 0.1mol of monocarboxylic acid requires V litres of O2 at dtc. and the reaction produces 0.3mol of CO2 and 0.2mol of H2O.
What is the value of V?
Explanation:
The balanced chemical equation of the reaction is
\(HCOOH+O_2 -> 2CO_2+2H_2O\\\)
1mol. of acid reacts with 1mol of \(O_2\) and produce 2mol. of CO2 and 2mol. of H2O.
Given,
0.1mol of acid requires how many litres of oxygen at STP?
0.1mol of acid requires 0.1mol of oxygen.
Since, 1mol of oxygen at STP occupies 22.4L of volume.
Hence, the volume occupied by 0.1mol of oxygen is -- 2.24L
Answer is:
The value of V is 2.24L.
why there is no reaction when aluminium is added into cold dilute hydrochloric acid
Answers
Answer:
When aluminum is added to cold dilute hydrochloric acid, there is no reaction because aluminum is a highly reactive metal, but it is protected by a thin oxide layer on its surface. This oxide layer is not easily dissolved by dilute hydrochloric acid, so the aluminum does not react with the acid. In order to react with the acid, a stronger acid such as sulfuric acid or nitric acid is needed to dissolve the oxide layer. Additionally, a higher concentration of hydrochloric acid is also needed to react with aluminum.
Another possible reason is that Aluminium metal react with hydrochloric acid to produce hydrogen gas and aluminum chloride salt, but the reaction is relatively slow and requires heat to speed it up. In cold dilute hydrochloric acid, the reaction rate is too slow to observe any visible change.
Answer:
There is a leyer of aluminium that prevents nothing from happening
Explanation:
Keep in mind that this reaction will not take place as soon as you add the piece of aluminium to the hydrochloric acid solution. That happens because the piece of aluminium is protected by a layer of aluminium oxide, Al2O3 , the same layer that protects aluminium from reacting with water
Question
What causes a water molecule to be polar?
A. The Oxygen atom is larger and stronger than the Hydrogen atoms so the electrons spend more of their time nearer the hydrogen
B. The Oxygen atom is smaller and weaker than the Hydrogen atoms so the electrons spend more of their time nearer the oxygen
C. The Oxygen atom is smaller and weaker than the Hydrogen atoms so the electrons spend more of their time nearer the hydrogen
D.The Oxygen atom is larger and stronger than the Hydrogen atoms so the electrons spend more of their time nearer the oxygen
Answers
Answer:
The correct option is D
Explanation:
Water molecules are polar because there structures are made up two sides; the positively charged side (the hydrogen side) and the negatively charged side (the oxygen side). The atoms in the water molecule are joined together by covalent bond - meaning there is sharing of electrons between the constituent atoms. The oxygen atom is bigger/larger (in size) and has a higher electronegativity (ability to attract electrons) hence, the electrons tend to spend more time around the oxygen than the hydrogen atom.
When performing an extraction with between an aqueous solution and organic solution what determines which layer ends up the bottom layer in the separatory funnel ?.
Answers
When performing an extraction between an aqueous solution and an organic solution or organic compound, density will determine the layer ending up at the bottom of the separation funnel.
In chemistry, a separatory funnel is a kind of funnel that separates two immiscible liquids.
It is a transparent funnel and when two immiscible liquids i.e aqueous and organic solution is poured into it, and then allowed to stand, after a while a distinguished layer is formed setting them apart from each other.
Due to the difference in density, the extraction becomes easier as the aqueous layer has a low density and stays at the top while the organic layer is at the bottom.
If you need to learn more about organic compounds click here:
https://brainly.com/question/19083306
#SPJ4
What is the name of Pb(NO3)2? Explain how you determined the bond type and the steps you used to determine the naming convention for the compound.
Answers
This chemical is known as lead (II) nitrate. It is an ionic assembly (salt compound) comprised of lead cations in the +2 oxidation state. With regard to the naming convention, each lead (II) cation is paired with two nitrate anions, each having a charge of -1.
What is a naming convention in Chemistry?Chemical nomenclature is a set of principles for naming chemical substances in a systematic manner. The International Union of Pure and Applied Chemistry designed and developed the most widely used nomenclature in the world (IUPAC).
The basic goal of chemical nomenclature is to guarantee that no ambiguity exists between a spoken or written chemical name and the chemical compound to which the name refers. Each chemical name should only relate to one substance.
It is required to indicate the charge of these cations or compounds containing these cations when identifying them. Ionic compounds are formed when cations and anions interact. The cation of an ionic compound is named first, followed by the anion. When writing their chemical formulae, they use the same format.
Learn more about naming conventions:
https://brainly.com/question/14326884
#SPJ1
A student makes an aqueous solution of sodium hydroxide. Which statement correctly identifies the two ions present in the solute of this mixture?
The sodium ion is negative, and the hydroxide ion is positive.
The sodium ion is positive, and the hydroxide ion is positive.
The sodium ion is positive, and the hydroxide ion is negative.
The sodium ion is neutral, and the hydroxide ion is neutral
Answers
The two ions present in the solution are:
1. Sodium ion, Na⁺
2. Hydroxide ion, OH¯
The correct answer to the question is the 3rd option. The sodium ion is positive, and the hydroxide ion is negative.
Ions are atoms or group of atoms which possess an electric charge.
Aqueous Sodium hydroxide, NaOH contains sodium ion and hydroxide ion.
The sodium ion is positive while the hydroxide ion is negative as illustrated below:
NaOH(aq) —> Na⁺(aq) + OH¯(aq)
From the illustration above, we can conclude that the correct answer to the question is the 3rd option. The sodium ion is positive, and the hydroxide ion is negative.
Please see attached photo for further details
Learn more: https://brainly.com/question/4414335

Cells are made of many parts, or , that help the cell do its job. Each cell organelle has a specific function, or
Answers
Answer:
Organelles; role.
Explanation:
A cell can be defined as the structural, fundamental, biological and functional unit of life. Cells are found in all living organisms because they are the basic unit of life. A unicellular organism refers to a living organism that possess a single-cell while a multicellular organism has many (multiple) cells. Generally, cells have the ability to independently replicate themselves. In a cell, the "workers" that perform various functions or tasks for the survival of the living organism are referred to as organelles. Some examples of cell organelles with their respective functions in all living organisms such as trees, birds, and bacteria include;
1. Nucleus : it controls all the activities taking place in the cell and the synthesis of proteins.
2. Mitochondria : it provides all the energy required in the cell by transforming energy forms.
3. Lysosomes : they are responsible for absorbing materials and breaking the materials taken in by the cells.
4. Chromosomes : they give sets of instructions for the synthesis of products.
5. Ribosomes : they are involved in the build up of proteins.
6. Endoplasmic Reticulum : this is where the ribosomes perform their tasks.
7. Cytoskeleton : they help to maintain and support the shape of the cells.
8. Vesicles : they ensure proteins are properly transported to the right and exact location.
9. Golgi apparatus : it prepares the protein for export by chemically tagging them.
10. Cell membrane : is the wall of the cell and typically controls what leaves and enters the cell.
Hence, cells are made of many parts, or organelles, that help the cell do its job. Each cell organelle has a specific function, or role.
the ability for bonds to take on an intermediate character in which the electrons are able to occupy different regions of a molecule at the same time is called hybridization. true false
Answers
True. The ability for bonds to take on an intermediate character in which the electrons are able to occupy different regions of a molecule at the same time is called hybridization.
What is hybridization ?When two atomic orbitals join to generate a hybrid orbital in a molecule, the energy of the individual atoms' orbitals is redistributed to give orbitals of equivalent energy. We refer to this process as hybridization. Hybridization occurs when the atomic orbitals of an atom are mixed together to form new, hybrid orbitals. These hybrid orbitals have properties that are intermediate between those of the original atomic orbitals, and they are able to bond with other atoms in different ways. Hybridization is important in the formation of chemical bonds and in the determination of the shape and properties of molecules.Hybridization is often used to explain the behavior of atoms in molecules, particularly when the bonding patterns and shapes of the molecules do not conform to the predictions of simple valence bond theory.To learn more about hybridization refer:
https://brainly.com/question/22765530
#SPJ1
write the empirical formula of at least four binary ionic compounds that could be formed from the following ions: mg2 , au3 , i-, o2-
Answers
The empirical formulas of four binary ionic compounds formed from the given ions are:
Magnesium iodide (MgI\(_{2}\))Gold(III) oxide (Au\(_{2}\)O\(_{3}\))Magnesium oxide (MgO)Gold(I) iodide (AuI)The empirical formula of a compound represents the simplest ratio of the elements present in it. In this case, we have three ions: Mg\(_{2}\)+, Au\(_{3}\)+, and I-. To form a binary ionic compound, the positive and negative charges of the ions need to balance.
For the first compound, magnesium iodide (MgI\(_{2}\)), two iodide ions (I-) are needed to balance the charge of one magnesium ion (Mg\(_{2}\)+).
For the second compound, gold(III) oxide (Au\(_{2}\)O\(_{3}\)), three oxide ions (O\(_{2}\)-) are required to balance the charge of two gold ions (Au\(_{3}\)+).
The third compound is magnesium oxide (MgO), where one oxide ion (O\(_{2}\)-) combines with one magnesium ion (Mg\(_{2}\)+).
Lastly, for gold(I) iodide (AuI), one iodide ion (I-) is required to balance the charge of one gold ion (Au+).
These empirical formulas represent the simplest combination of ions in these ionic compounds.
You can learn more about empirical formulas at
https://brainly.com/question/1603500
#SPJ11
When a malonic ester synthesis is performed using excess base and 1,4-dibromobutane as the alkyl halide, an intramolecular reaction occurs, and the product contains a ring. Draw the product of this process:
Answers
when a malonic ester synthesis is performed using excess base and 1,4-dibromobutane as the alkyl halide, an intramolecular reaction occurs, forming a cyclic compound. The product of this reaction is 3-bromocyclopentanecarboxylic acid.
Malonic ester synthesis is a useful method for the synthesis of carboxylic acids and ketones. It involves the reaction of diethyl malonate (also known as malonic ester) with an alkyl halide in the presence of a strong base such as sodium ethoxide or sodium hydride. The alkyl group of the alkyl halide replaces one of the ester groups of diethyl malonate, forming a new carbon-carbon bond. The resulting compound is then hydrolyzed with acid to form the final product.
In the case of using 1,4-dibromobutane as the alkyl halide and excess base, an intramolecular reaction occurs. This means that the reaction takes place within the same molecule rather than between two separate molecules. The reaction involves the attack of one of the ester groups on the α-carbon of the other ester group, forming a cyclic compound. This intramolecular reaction is favored because it forms a more stable six-membered ring.
The product of this reaction is 3-bromocyclopentanecarboxylic acid.
The six-membered ring is formed between the α-carbon and the carbonyl carbon of the same ester group. The bromine atom is located on the α-carbon of the other ester group.
In summary, when a malonic ester synthesis is performed using excess base and 1,4-dibromobutane as the alkyl halide, an intramolecular reaction occurs, forming a cyclic compound. The product of this reaction is 3-bromocyclopentanecarboxylic acid.
To know more about malonic acid, refer
https://brainly.com/question/27755540
#SPJ11
the pressure gradient (δp) driving blood flow through the systemic circuit is equated to the
Answers
The pressure gradient (δp) driving blood flow through the systemic circuit is equated to the difference between the mean arterial pressure (MAP) and the mean venous pressure (MVP).
This pressure gradient represents the force that propels blood flow from the arteries, through the capillaries where exchange of nutrients and waste occurs, and into the veins that return blood back to the heart.
The MAP is the average pressure exerted by blood on the walls of arteries during one cardiac cycle and is usually around 90 mmHg in a healthy adult at rest. The MVP is the average pressure in the venous system and is typically around 10 mmHg in a healthy adult.
Therefore, the pressure gradient (δp) can be calculated as:
δp = MAP - MVP
Learn more about blood flow : https://brainly.com/question/31110834
#SPJ11
which statement describes how the binary ionic compound cacl2 is named?
Answers
Constituent elements of bronze
Answers
Answer:
Bronze is an alloy.
Explanation:
Bronze is an alloy consisting primarily of copper, commonly with about 12 to 12.5%tin and often with the addition of other metal such as aluminium, nickel, manganese and zinc.
When heated, KClO3 decomposes into KCl and O2. 2KClO3⟶2KCl+3O2 If this reaction produced 13.2 g KCl, how many grams of O2 were produced?
Answers
Answer:
8.5gm O2 produced
Explanation:
When heated, KClO3 decomposes into KCl and O2. 2KClO3⟶2KCl+3O2 If this reaction produced 13.2 g KCl, how many grams of O2 were produced?
for every 2 moles of KCl produced, 3 moles of O2 are produced
Mol weight of KCl =39+35.5=74.5gm
13.2 gm KCl = 13.2/74.5 = 0.177 moles
this will make (3/2) X 0.177 = 0.2655 moles of O2
O2 mol wt is 32 0.2655 X32 = 8.5gm O2 produced
Using the table of average bond energies, determine the total bond energy for the reactants in the combustion of ethene: C2H4 + 3 O2 --> 2 CO2 + 2 H2O

Answers
Explanation:
Hydrogen bonds with carbon 4 times, carbon bonds with itself once, and oxygen bonds with itself 3 times.
Energy of reactants:
E = 4 * 413 + 347 + 3 * 495
= 3484kJ/mol
Answer:
Energy transferred in = 3484kJ/mol
Abbreviations along with the explanation are shown. Which set used in chemical equations is correct?
O (g). grams
O (aq), dissolved in water
O (1), liters
O, a gas is evolved
Answers
Answer:
B and D
Explanation:
Answer D, look like you're missing (g) which stands for gas
so answer B (which is aq) and answer D (which is g) are correct.
Grams and litters are not used in the chemical equations but used in calculation of the quantities of reactants and products.