Answers
Answer:
Kinetic energy
Explanation:
Fireworks convert chemical energy into kinetic energy to send them flying upward. Hope it is correct!
Related Questions
2. Which state of matter is characterized by particles that are close to each other but are not arranged in a definite pattern?
A)liquid
B)plasma
C)solid
D)gas
Answers
Answer:
Solid
Explanation:
Cus its solid, take a brick for example. It's hard and has no space unlike liquid or gas.
0.2g of sand in two-third in liter of ethanol . What is the concentration in g per dm cube
Answers
The mass concentration of sand in the ethanol solution is 0.299 g/dm³.
What is the concentration in grams per dm³?To find the concentration in grams per cubic decimeter (g/dm³), we first need to convert the volume from liters to cubic decimeters (dm³). Since 1 liter is equal to 1 cubic decimeter, we can directly convert the volume.
Given:
Mass of sand = 0.2 g
Volume of ethanol = two-thirds liter
Converting volume to dm³:
1 liter = 1 cubic decimeter
two-thirds liter = (2/3) cubic decimeter = 0.67 dm³ (rounded to two decimal places)
Now we can calculate the concentration in g/dm³ by dividing the mass of sand by the volume in dm³:
Concentration = Mass / Volume
Concentration = 0.2 g / 0.67 dm³
Concentration ≈ 0.299 g/dm³ (rounded to three decimal places)
Learn more about mass concentration at: https://brainly.com/question/23437000
#SPJ1
I need the answer for 14
(It for earth science)

Answers
Answer:
Fg=mg
Explanation:
Describe the advantages of the hydrogen-rich fuel cell when compared to the conventional electrochemical cells such as lead-acid battery. (4)
Answers
The hydrogen-rich fuel cell offers advantages in terms of efficiency, environmental impact, operating time, refueling speed, weight, size, and lifespan when compared to conventional electrochemical cells like the lead-acid battery.
The hydrogen-rich fuel cell offers several advantages over conventional electrochemical cells like the lead-acid battery. Here are some of the key advantages:
1. Higher Efficiency: Hydrogen fuel cells have higher energy conversion efficiencies compared to lead-acid batteries. Fuel cells can convert chemical energy directly into electrical energy with minimal loss, while lead-acid batteries have inherent energy losses due to factors such as internal resistance and heat dissipation.
2. Clean and Environmentally Friendly: Hydrogen fuel cells produce electricity through the reaction of hydrogen and oxygen, with water being the only byproduct. They do not produce harmful emissions or contribute to air pollution, making them a cleaner and more sustainable power source compared to lead-acid batteries, which require the use of chemicals like sulfuric acid.
3. Longer Operating Time: Fuel cells have longer operating times compared to lead-acid batteries. Lead-acid batteries have a limited capacity and need to be recharged frequently, while fuel cells can continuously generate electricity as long as there is a supply of hydrogen.
4. Faster Refueling: Refueling a fuel cell is faster compared to recharging a lead-acid battery. Fuel cells can be refueled by replenishing the hydrogen supply, which can be done relatively quickly. In contrast, lead-acid batteries require a longer time to recharge, typically hours, depending on the battery's capacity and charging rate.
5. Lighter Weight and Compact Size: Hydrogen fuel cells have a higher energy density compared to lead-acid batteries, meaning they can store more energy in a smaller and lighter package. This makes fuel cells more suitable for applications where weight and space are critical, such as in portable devices or electric vehicles.
6. Longer Lifespan: Fuel cells generally have a longer lifespan compared to lead-acid batteries. Lead-acid batteries can experience degradation over time due to factors like sulfation, which can reduce their overall capacity and lifespan. Fuel cells, on the other hand, can provide consistent performance over an extended period with proper maintenance.
These advantages make fuel cells a promising technology for various applications, including transportation, stationary power generation, and portable electronics.
for more questions on fuel cell
https://brainly.com/question/14122421
#SPJ8
What was the niche of the lion king
Answers
Answer:
Known as the "king of the jungle" or the "king of beasts," lions are at the top of the food chain, and they hunt and eat other animals to survive. Their niche in the ecosystem allows them to help with other animal population control and prevent the spread of disease.
Explanation:
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
An clement X has 2 electrons in K shell, 8 electrons in L shell and 5 electrons in i Size of X ion is greater than that of X atom though both contain the same protons. Give reason. ii) Write down the formula of one of the compounds of X where X is in -3 oxidation.
Answers
Answer:
i) The size of X ion is greater than that of X atom even though both contain the same number of protons because the ion has fewer electrons compared to the atom. When an atom forms an anion (negative ion), it gains electrons, which causes increased electron-electron repulsion. This repulsion causes the electron cloud to expand, and as a result, the ion becomes larger than the neutral atom.
In the case of element X, when it forms an ion with a -3 charge, it will gain 3 more electrons, increasing the total number of electrons to 18. This will cause the size of the X ion to be larger than the neutral X atom.
ii) To determine the compound of X in the -3 oxidation state, we first need to determine the element's identity. We know that X has 15 electrons in total (2 in the K shell, 8 in the L shell, and 5 in the M shell). Therefore, X has an atomic number of 15, which corresponds to phosphorus (P).
Since phosphorus is in the -3 oxidation state, it gains 3 electrons and becomes P^3-. To form a compound, we need a cation that can balance the negative charge. A common example is aluminum (Al), which has a +3 charge (Al^3+). When phosphorus and aluminum combine, they form the compound aluminum phosphide with the formula AlP.
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
How many molecules are in
5.657g H2SO4?
Answers
There are approximately 3.47 x 10²² molecules in 5.657g H₂SO₄.
To calculate the number of molecules in 5.657g H₂SO₄, we need to use the Avogadro's number and the molar mass of H₂SO₄.
The molar mass of H₂SO₄ is 98.079 g/mol.
We need to calculate the number of moles of H₂SO₄:
Number of moles = mass/molar mass
= 5.657g / 98.079 g/mol
= 0.05767 mol.
Then, we can use Avogadro's number, which is 6.022 x 10²³ molecules/mol, to find the number of molecules:
Number of molecules = number of moles x Avogadro's number
= 0.05767 mol x 6.022 x 10²³ molecules/mol
= 3.47 x 10²² molecules
To calculate the number of molecules in a given sample of a substance, you need to use the Avogadro's number, which is 6.022 x 10²³ molecules/mol. This means that one mole of a substance contains 6.022 x 10²³ molecules.
We are given the mass of H₂SO₄, which is 5.657 g. To calculate the number of molecules, we first need to determine the number of moles of H₂SO₄ in the given sample. The molar mass of H₂SO₄ is 98.08 g/mol. So, the number of moles of H₂SO₄ can be calculated as follows:
moles = mass / molar mass
moles = 5.657 g / 98.08 g/mol
moles = 0.0576 mol
Now, we can use the Avogadro's number to determine the number of molecules of H₂SO₄ in 0.0576 moles:
number of molecules = moles x Avogadro's number
number of molecules = 0.0576 mol x 6.022 x 10²³ molecules/mol
number of molecules = 3.47 x 10²² molecules
As a result, in 5.657 g of the material, there are roughly 3.47 x 1022 molecules of H₂SO₄.
To know more about the Molecules, here
https://brainly.com/question/11488454
#SPJ1
Generally, how do atomic masses vary throughout the periodic table of the elements?
They increase from left to right and top to bottom.
O They increase from left to right and bottom to top.
O They increase from right to left and top to bottom.
O They increase from right to left and bottom to top.
Answers
Answer:
They increase from left to right and top to bottom.
Answer:
They increase from left to right and top to bottom.
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
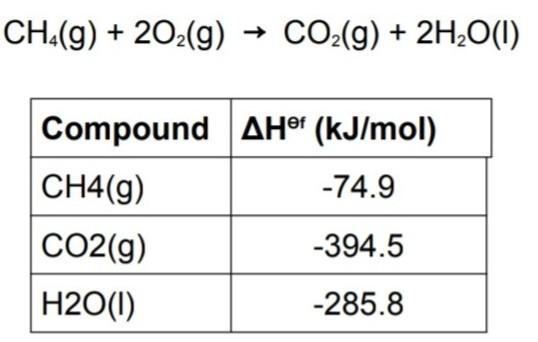
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
4. How many grams is 3 moles of H₂O?
Answers
Answer:
1.67
Explanation:
Mass÷mr=moles
3÷18=1.67
The number of nitrogen atoms in one mole of nitrogen gas are...
Answers
Explanation:
The number of nitrogen atoms in one mole of nitrogen gas are 6.02214179×1023 nitrogen atoms.
Hope this helps...
how many bond can boron make without hybridization
Answers
Without hybridization, boron can form 4 bonds
What is hybridization?Hybridisation is phenomenon of combining two atomic orbitals to give a new degenerate hybrid orbital which have same energy levels. Hybridization increases the stability of bond formation than unhybridized orbitals. We can predict the shape of molecules by its hybridization.
With hybridization , boron can form 3 bonds.
Hence, without hybridization, Boron donates the lone pair of electrons to form the fourth bond. In addition to that Boron is a second period element hence, which makes it small in size and d-orbitals are unavailable as well. Hence, Boron can only form 4 bonds with hybridization.
learn more about hybridization from
https://brainly.com/question/22765530
#SPJ1
Getting a vaccine is safer than taking medication or undergoing surgery. True or false I
Answers
Answer:
True; you really think getting surgery is safer than getting a tiny shot? I think not.
What is the difference between the speed of an object, the velocity of an object and the
acceleration of an object?
Answers
Speed is the rate of change of distance(basically how much distance(m) has been covered in a particular time(s)). Velocity is the rate of change of displacement( change of distance in a particular direction with respect to time) , and acceleration is the rate of change of velocity per unit of time.
For a particular reaction at 135.4
°C, Δ=−775.41 kJ/mol
, and Δ=817.91 J/(mol⋅K)
.
Calculate ΔG for this reaction at 12.7
°C.
Answers
Answer:
\(\Delta G=-675.38 \frac{kJ}{mol}\)
Explanation:
Hello!
In this case, for this problem, it is possible to use the thermodynamic definition of the Gibbs free energy:
\(\Delta G=\Delta H-T\Delta S\)
Whereas G, H and S can be assumed as constant over T; thus, we can calculate H at 135.4 °C:
\(\Delta H=\Delta G+T\Delta S\\\\\Delta H=-775.41\frac{kJ}{mol}+(135.4+273.15)K*(0.81791\frac{kJ}{mol*K} )\\\\\Delta H=-441.58\frac{kJ}{mol}\)
Now, we can calculate the Gibbs free energy at 12.7 °C as shown below:
\(\Delta G=-441.58\frac{kJ}{mol} -(12.7+273.15)K*0.81791\frac{kJ}{mol*K}\\\\\Delta G=-675.38 \frac{kJ}{mol}\)
Best regards!
Place a test tube in the test tube rack, and label it 3. Then follow these steps using your prepared solution:
1. Use the pipette to remove copper(II) sulfate solution from the volumetric flask, and measure out 10 milliliters in the
graduated cylinder. Transfer the 10 milliliters of solution from the graduated cylinder to test tube 3.
2. Record the temperature of the solution in the table provided.
3. Measure 0.25 gram of zinc powder into a weighing boat.
4. Pour the zinc powder into test tube 3.
5. Measure the final temperature of the solution in test tube 3. Watch the thermometer for a couple of minutes, and
record the highest temperature it reaches.
6. Calculate and record the difference of the initial and final temperatures in the table.
B I y x² X, 10pt
AVV EEEEE 図 √ 田
Measurement
Initial temperature (°C)
Final temperature (°C)
Temperature change (°C)
V
Answer
Answers
Based on the provided steps, we are conducting an experiment involving copper(II) sulfate solution and zinc powder. We are measuring the temperature change that occurs when zinc reacts with copper(II) sulfate.
Measurement:
Initial temperature (°C): This is the temperature of the copper(II) sulfate solution before adding zinc powder. Use a thermometer to measure and record this temperature.
Final temperature (°C): This is the highest temperature reached by the solution after adding the zinc powder and allowing the reaction to occur. Watch the thermometer for a couple of minutes and record the highest temperature observed.
Temperature change (°C): Calculate the difference between the initial and final temperatures. Subtract the initial temperature from the final temperature and record the result as the temperature change.
Follow the steps provided to carry out the experiment and record the corresponding measurements in the table. Make sure to use the pipette to transfer 10 milliliters of copper(II) sulfate solution to test tube 3, then add 0.25 grams of zinc powder to the test tube.
Monitor the temperature using a thermometer and record the initial and final temperatures accurately. Finally, calculate the temperature change by subtracting the initial temperature from the final temperature and record the value in the table.
For more such questions on copper(II) sulfate visit:
https://brainly.com/question/31259348
#SPJ8
There are two isotopes of an unknown element, X-19 and X-21. The abundance of X-19 is 12.01%. Now that you have the contribution from the X-19 isotope (2.282) and from the X-21 isotope (18.48), what is the average atomic mass (in amu) of this element using four significant figures
Answers
Answer: The average atomic mass of X is 16.53
Explanation:
Mass of isotope X-19 = 2.282
% abundance of isotope X-19 = 12.01% = \(\frac{12.01}{100}=0.1201\)
Mass of isotope X-21 = 18.48
% abundance of isotope X-21 = (100-12.01)% = \(\frac{100-12.01}{100}=0.8799\)
Formula used for average atomic mass of an element :
\(\text{ Average atomic mass of an element}=\sum(\text{atomic mass of an isotopes}\times {{\text { fractional abundance}})\)
\(A=\sum[(2.282\times 0.1201)+(18.48\times 0.8799)]\)
\(A=16.53\)
Therefore, the average atomic mass of X is 16.53
Calculate the molarity of 1 L of water at 20 °C given a density of 0.9982 g/mL.
Answers
Answer:
I have the same thing
Explanation:
How many atoms are in 29.79 g of copper (Cu)?
Answers
Answer:
2.80×10^23atoms
Explanation:
1mol of any element contains 6.02×10^23 atoms (Avogadro's constant)
1mole of copper is 64g
64g contain 6.02×10^23 atoms
29.78g will contain X atoms
cross multiply29.79g × 6.02×10^23atoms= 64g×Xatom
divide both by 64gX= 29.79g×6.02×10^23atoms/64g
X=2.80×10^23atoms
3. How many grams of oxygen are required to completely react with 240g of C₂H6?
Answers
Approximately 766.08 grams of oxygen are required to completely react with 240g of C₂H₆.
To determine the amount of oxygen required to completely react with 240g of C₂H₆ (ethane), we need to set up a balanced chemical equation for the combustion of ethane.
The balanced equation for the combustion of ethane is as follows:
C₂H₆ + O₂ → CO₂ + H₂O
From the balanced equation, we can see that the stoichiometric ratio between C₂H₆ and O₂ is 1:3. This means that for every one mole of C₂H₆, three moles of O₂ are required for complete combustion.
To calculate the amount of oxygen required, we need to convert the given mass of C₂H₆ to moles using its molar mass, and then use the stoichiometric ratio to determine the moles of O₂ required. Finally, we can convert the moles of O₂ back to grams using the molar mass of oxygen.
The molar mass of C₂H₆ is calculated as follows:
(2 x atomic mass of carbon) + (6 x atomic mass of hydrogen)
(2 x 12.01 g/mol) + (6 x 1.01 g/mol) = 30.07 g/mol
Now, we can proceed with the calculation:
Calculate the moles of C₂H₆:
moles of C₂H₆ = mass of C₂H₆ / molar mass of C₂H₆
moles of C₂H₆ = 240 g / 30.07 g/mol ≈ 7.98 mol
Determine the moles of O₂ using the stoichiometric ratio:
moles of O₂ = moles of C₂H₆ x (3 moles of O₂ / 1 mole of C₂H₆)
moles of O₂ = 7.98 mol x 3 ≈ 23.94 mol
Convert moles of O₂ to grams:
mass of O₂ = moles of O₂ x molar mass of O₂
mass of O₂ = 23.94 mol x 32.00 g/mol = 766.08 g
For more such questions on oxygen visit:
https://brainly.com/question/28009615
#SPJ8
what is 1.602176*10-¹⁹/ 1.758820*10¹¹C kg-¹
with proper explanation.
or expanded form
Answers
Answer:
0.00000000910938;
9.109380 * 10^-9
Explanation:
Given the question :
Given :
1.602176*10-¹⁹/ 1.758820*10¹¹
The numbers are expressed in the form:
A * 10^b ; Where;
A = Coefficient ; 10 = base ; b = exponent
The Coefficients can be divided directly as :
1.602176 / 1.758820 = 0.9109380
Using the division law of indices, the exponents acn be worked out as follows since they have the same base :
10^-19 / 10^-11 = 10^(-19 - (-11)) = 10^(-19+11) = 10^-8
Thus, we have ;
0.9109380 × 10^-8
In standard form:
9.109380 * 10^-9
In Expanded form:
10^-8 = 0.00000001
0.9109380 * 0.00000001 = 0.00000000910938
0.000000009 +
0.0000000001 +
0.00000000000 +
0.000000000009 +
0.0000000000003 +
0.00000000000008
__________________
0.00000000910938
___________________
A sample of ammonia, NH3, has a mass of 78.25 g. Calculate the number of ammonia molecules in the sample.
number of molecules:
Answers
There are approximately \(2.76 * 10^{24\) ammonia molecules in the given sample.
To calculate the number of ammonia molecules in the sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia \((NH_3)\) can be calculated by adding up the atomic masses of nitrogen (N) and hydrogen (H):
Molar mass of \(NH_3\) = (1 x atomic mass of N) + (3 x atomic mass of H)
= (1 x 14.01 g/mol) + (3 x 1.01 g/mol)
= 14.01 g/mol + 3.03 g/mol
= 17.04 g/mol
Now, we can calculate the number of moles of ammonia in the sample using the formula:
Number of moles = Mass of the sample / Molar mass
Number of moles = 78.25 g / 17.04 g/mol
≈ 4.5865 mol (rounded to four decimal places)
Finally, we can use Avogadro's number, which represents the number of particles (atoms, molecules, etc.) in one mole of a substance. Avogadro's number is approximately \(6.022 * 10^{23\) particles/mol.
Number of ammonia molecules = Number of moles x Avogadro's number
Number of ammonia molecules ≈ 4.5865 mol x (\(6.022 * 10^{23\) molecules/mol)
≈ \(2.76 * 10^{24\) molecules (rounded to two significant figures)
Therefore, the provided sample contains roughly \(2.76 * 10^{24\) ammonia molecules.
Learn more about moles on:
https://brainly.com/question/24748125
The number of ammonia molecules in the sample is approximately 2.764 x \(10^{24}\) molecules.
To calculate the number of ammonia molecules in a given sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia (NH3) is calculated as follows:
Molar mass of N = 14.01 g/mol
Molar mass of H = 1.01 g/mol
Total molar mass of NH3 = 14.01 g/mol + (3 * 1.01 g/mol) = 17.03 g/mol
Now, we can calculate the number of moles of ammonia in the sample:
Number of moles = Mass of sample / Molar mass of NH3
Number of moles = 78.25 g / 17.03 g/mol = 4.594 moles
Next, we use Avogadro's number, which states that there are 6.022 x \(10^{23}\) molecules in one mole of a substance.
Number of molecules = Number of moles * Avogadro's number
Number of molecules = 4.594 moles * 6.022 x \(10^{23}\) molecules/mol = 2.764 x \(10^{24}\) molecules
Therefore, there are approximately 2.764 x \(10^{24}\) ammonia molecules in the given sample of 78.25 g.
Know more about Avogadro's number here:
https://brainly.com/question/1513182
#SPJ8
A vessel of volume 100ml contains 10% of oxygen and 90% of an unknown gas. The gases diffuses in 86 second through a small hole of vessel.
If pure oxygen under similar
conditions and diffuses in 75 second, find the molecular weight of unknown gas?
Answers
The molecular weight of unknown gas : 23.46 g/mol
Further explanationGiven
A vessel contains 10% of oxygen and 90% of an unknown gas.
diffuses rate of mixed gas = 86 s
diffuses rate of O₂ = 75 s
Required
the molecular weight of unknown gas (M)
Solution
The molecular weight of mixed gas :(M O₂=32 g/mol)
\(\tt 0.1\times 32+0.9\times M=3.2+0.9M\)
Graham's Law :
\(\tt \dfrac{r_{O_2}}{r_{mixed~gas}}=\sqrt{\dfrac{M_{mixed}}{M_{O_2}} }\\\\\dfrac{75}{86}=\sqrt{\dfrac{3.2+0.9M}{32} }\\\\0.76=\dfrac{3.2+0.9M}{32}\\\\24.32=3.2+0.9M\\\\21.12=0.9M\rightarrow M=23.46~g/mol\)
Based on the Law of Conservation of Mass,
what mass of products form when baking
soda decomposes?
NaHCO3 → Na₂CO3 + H₂O + CO₂
25.00 g
Give your answer to the correct number of
significant figures.
(g) Sodium Chloride
?g
Enter
Answers
Based on the Law of Conservation of Mass, the mass of products form when baking soda decomposes is 168 g/mole.
What is law of conservation of mass?Law of conservation of mass is defined as chemical reactions and physical changes cannot build or remove mass in an isolated system. The mass of the reactants and products in a chemical reaction must equal each other according to the law of conservation of mass.
\(\rm 2NaHCO_3 \rightarrow Na_2CO_3 + H_2O + CO_2\)
Mass of baking soda = 2 x molar mass
Molar mass of NaHCO₃ = 84.007 g/mole
Mass of baking soda = 2 x 84.007 g/mole
Mass of baking soda = 168.014 g/mole ≅ 168 g/mole
Thus, based on the Law of Conservation of Mass, the mass of products form when baking soda decomposes is 168 g/mole.
To learn more about Law of Conservation of Mass, refer to the link below:
https://brainly.com/question/28711001
#SPJ1
if 14.0 g of sodium sulfide are reacting how many molecules of sodium chloride will be produced

Answers
Explanation:
If 14.0 g of sodium sulfide are reacting, how many molecules of sodium chloride will be produced?
3 Na₂S + 2 AICI36 NaCl + Al2S3
1.08 x 10^23
2.16 x 10^23
2.16 x 10^-23
5.97 x 10^-25
Choose the equation below that is balanced correctly.
S8 +24 028 SO3
S8+ 12 0₂8 SO3
6 S8+8 026 SO3
2 S8 +3 022 SO3
Answers
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
What is the balanced chemical equation?Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products.
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is determined as;
2S₈ + 16O₂ → 16SO₃
From the reactants side we can see that sulfur is 16 and also 16 in the product side. The number of oxygen in the reactant side is 32 and also 32 in the product side.
Thus, the balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
Learn more about balanced chemical equation here: https://brainly.com/question/26694427
#SPJ1
What is the balanced equation for the redox reaction between zinc and hydrochloric acid (HCl) that forms zinc(II) ions and hydrogen gas? A. 2Zn + HCl + H+ Zn2Cl + H2 B. Zn + 2H+ 2Zn2+ + H2 C. 2Zn + 2H+ 2Zn2+ + H2 + 2e- D. Zn + 2H+ 2Zn2+ + H2 + 2e-
Answers
The balanced equation for the redox reaction between zinc and hydrochloric acid (HCl) that forms zinc(II) ions and hydrogen gas is as follows:
Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
What is a redox reaction?A redox reaction is a type of chemical reaction in which some of the atoms have their oxidation number changed.
According to this question, the redox reaction between zinc and hydrochloric acid (HCl) forms zinc(II) ions and hydrogen gas. The balanced equation is as follows:
Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
Learn more about redox reaction at: https://brainly.com/question/13293425
#SPJ1