Find the kinetic energy of a 5kg bowling ball that is traveling 2 meters per second
Answers
Explanation:
KE = ½ mv²
= ½ x 5 x 2² joule
= 10 joule
Related Questions
Oxygen molecules absorbed by cells: the athlete's result will be
Answers
Oxygen molecules absorbed by cells: the athlete's result will be higher than the normal healthy body's result.
What is oxygen molecules?
Two oxygen atoms are bound together by a covalent connection to form the diatomic molecule known as molecular oxygen (O2). Since so many different types of creatures need molecular oxygen for breathing, it is crucial for life. Combustion of fossil fuels also requires it.
Because of its high chemical reactivity, molecular oxygen frequently reacts with other elements and compounds to generate oxides. We rely on plant photosynthesis to replace the molecular oxygen in the atmosphere; if photosynthesis were to cease, atmospheric oxygen levels would gradually fall to almost zero.
It is significant therapeutically since all creatures, including humans, breathe molecular oxygen and need it for metabolism. In addition to inhaling gas, molecular oxygen is used medically in oxygen therapy and hyperbaric chambers.
Learn more about Oxygen molecules from given link
https://brainly.com/question/17150869
#SPJ4
If the crucible and precipitate is not cool when the mass is determined, will the calculuated percent silver be too high or too low?
Answers
As the metal scale will shrink as a result of the heat, making the measurement inaccurate, it would be too high.
What is precipitate formation in a reaction ?A "chemical reaction happening in an aqueous solution when two ionic bonds combine, yielding the creation of an insoluble salt" is what is meant by the phrase "precipitation reaction." Precipitates are the insoluble salts created during precipitation processes. Precipitation reactions are often double displacement events that result in the formation of the precipitate, a solid form of residue. The creation of insoluble salts that precipitate out of the solution results from these reactions when two or more solutions with various salt concentrations are mixed.The chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride is precipitated out, is one of the greatest examples of precipitation reactions. This is the precipitation reaction's byproduct, the insoluble salt. Below is given the chemical equation for this precipitation process.
AgNO3(aqueous) + KCl(aqueous) → AgCl(precipitate) + KNO3(aqueous)
To view more about precipitate, refer to:
https://brainly.com/question/2263524
#SPJ4
Which is expected to have the largest dispersion forces?
a) C3H8
b) C12H26
c) F2
d) BeCl2
Answers
B: \(C_{12} H_{26}\) is expected to have the largest dispersion forces.
Dispersion force is a type of intermolecular force that acts between normally electrically symmetric atoms and molecules; that is, the electrons and atoms are symmetrically distributed with respect to the nucleus.
The strength of dispersion forces usually increases with the number of electrons in the atom or molecule. In the given case, \(C_{12}H_{26}\) contains a lot more electrons in comparison to \(C_{3} H_{8}\),\(F_{2}\), and \(BeCl_{2}\). Therefore, \(C_{12} H_{26}\) is expected to have the largest dispersion forces.
You can learn more about dispersion forces at
https://brainly.com/question/1455074
#SPJ4
answer
2. Describe the hydrologic cycle (use a graph to support your description). 3. Define the science of hydrology. 4. Define the watershed and catchment approach
Answers
The hydrologic cycle is the continuous movement and circulation of water on Earth. It involves the processes of evaporation, condensation, precipitation, infiltration, runoff, and transpiration.
The energy of the Sun powers the hydrologic cycle, often known as the water cycle. Ice sublimes, or changes instantly from a solid to a gas, when the sun warms the ocean's surface and other surface water. These solar-powered processes release water vapor, or vaporized water, into the atmosphere.
Water vapor in the atmosphere gradually forms clouds that eventually collapse to form precipitation, such as rain or snow. It is possible for precipitation to re-evaporate, flow over the surface, infiltrate into the soil, or percolate—sink deeper—into the ground as it strikes the Earth's surface.
The scientific study of water on Earth's surface, in its atmosphere, and underneath is known as hydrology. It encompasses the movement, distribution, and properties of water in its various forms. The watershed and catchment approach refers to the study and management of water resources based on the geographic area where water drains into a common outlet, such as a river, lake, or ocean.
To know more about hydrologic cycle, refer:
https://brainly.com/question/25796102
#SPJ4

which is not true for combustion reactions?
a. they always occur in the presence of oxygen
b. they produce carbon dioxide and water
c. they usually require a lot of energy to form products
d. a candle burning is an example of one
Answers
Answer:
They usually require a lot of energy to form products
Explanation:
Energy may not be reasonable source.Oxygen and hydrocarbon is enough to produce the products .Petroleum products, Carbon and hydrocarbons are also necessary
What is produced at the anode in the electrolysis of molten CuF2? O A. F2 O B. H2 O C. cu D.02
Answers
The produced at the anode in the electrolysis of molten CuF2 is A. F₂.
During electrolysis, CuF₂ will separate into Cu₂⁺ cations and F⁻anions. Cu₂⁺ will be reduced to copper metal, which will deposit on the cathode, whereas F⁻ anions will be oxidized to fluorine gas on the anode in the electrolysis of molten CuF₂. Hence, the answer is option A. Fluorine gas (F₂) is generated at the anode in the electrolysis of molten CuF₂.
Therefore, during the electrolysis of molten CuF₂, Cu₂⁺ is reduced to copper metal, which deposits on the cathode, and F⁻ anions are oxidized to fluorine gas on the anode, which is produced at the anode. The chemical reactions taking place during electrolysis of CuF₂ are given below: At the cathode, Cu₂⁺ cations get reduced to copper metal. Cu₂⁺ + 2e⁻ ⟶ Cu. At the anode, F⁻ anions get oxidized to fluorine gas. 2F⁻ ⟶ F₂ + 2e⁻. Therefore, option A is correct.
Learn more about electrolysis at:
https://brainly.com/question/12994141
#SPJ11
A student adds 4. 00 g of dry ice (solid co2) to an empty bal- loon. What will be the volume of the balloon at stp after all the dry ice sublimes (converts to gaseous co2)?.
Answers
The volume of the balloon at stp after all the dry ice sublimes is as follows:
We must first determine the moles of dry ice. Dry ice's molecular weight is 44 g/mol.
Mole of dry ice= mass of dry ice/molar mass of dry ice=4/44=0.0909mol
We now need to determine the dry ice's volume. As is common knowledge, 22.4 L of gas are contained in 1 mole of a substance.
As 22.4 L of gas are contained in 1 mole of dry ice. As a result, 0.0909 mole of dry ice holds 2.04 L (or 0.0909 mole x 22.4 L) of gas.
Consequently, the balloon's volume at STP is 2.04L
to know more about dry ice visit
https://brainly.com/question/13972158
#SPJ4
Balancing Chemical Reactions Worksheet A glow stick is a popular toy and safety device. To use a glow stick, you bend a small flexible plastic tube to break a small glass capsule inside, at which point the stick begins to glow. How do you think this works
Answers
Answer:
Concept of chemi-fluorescence
Explanation:
A glow stick usually consists of two chemicals in a larger plastic tube: , a base catalyst (mostly sodium salicylate), and a suitable dye (sensitizer, or fluorophor). This creates an exergonic reaction when mixed together.
When a glow stick is bent, the flurophor which is a chemical that easily re-emits light upon excitation in smaller capsules is released into the other substance, there by causing it to emit radiation/light in the uv-visible region. The brightness and longevity of the glow stick is determined by varying the concentration of these chemicals.
I hope this explanation clarifies things.
a
Scientists have observed that the average surface temperature of the Earth has been increasing. This increase has
affected the oceans. Glaciers and ice sheets melt, and water from this melting process becomes part of the oceans.
The increasing temperature of the Earth's surface also warms ocean waters. As a result, the volume of the oceans
expands. These two events affect sea level.
What would happen to sea level if the average surface temperature trend that was observed since the 1960s
continues?
A) Sea level will rise, then will suddenly fall.
B) Sea level will remain the same.
C) Sea level will continue to fall.
D) Sea level will continue to rise.
Please select the best answer from the choices provided
O A
OB
Save and Exit
Next
Submit
Mark this and return

Answers
Answer:
D) Sea level will continue to rise
The United Arab Emirates (UAE) is a major producer of crude oil. One barrel of crude oil is equal to 159 liters. The composition of crude oil varies, but on average, it contains 84% carbon and 14% hydrogen by mass. If the combustion of one barrel of crude oil produces 322 kg of carbon dioxide and 126 kg of water, what is the mass percentage of carbon in the carbon dioxide?
Answers
The mass percentage of carbon in the carbon dioxide is 27.0%.
One barrel of crude oil produces 322 kg of carbon dioxide, which is composed of carbon and oxygen. From the balanced chemical equation for the combustion of hydrocarbons, we know that one mole of carbon produces one mole of carbon dioxide. The molar mass of carbon is 12.01 g/mol, and the molar mass of carbon dioxide is 44.01 g/mol. Therefore, the mass percentage of carbon in carbon dioxide is:
(12.01 g C / 44.01 g CO₂) x 100% = 27.0%This means that for every 100 g of carbon dioxide produced from the combustion of crude oil, 27 g of it is carbon. Since crude oil contains 84% carbon by mass, this suggests that the carbon in the crude oil is not being fully converted to carbon dioxide during combustion, and that other carbon-containing compounds are being produced as well.
To learn more about mass percentage, here
https://brainly.com/question/31309409
#SPJ1
A cup of marbles and water is a homogenous mixture / heterogenous mixture. Why?
Answers
Answer:
Another example of a homogeneous mixture is salt water. The rock in Figure above is an example of a heterogeneous mixture. A heterogeneous mixture varies in its composition
Explanation:
He
4
+A
u
197
→A
u
197
+He
4
>α-Particle α-particle Goldshow how to calculate the q value for this nuclear reaction.
Answers
The q value for this nuclear reaction is zero, indicating no energy release or absorption.
The q value for this nuclear reaction can be calculated by subtracting the sum of the masses of the reactants from the sum of the masses of the products and converting it to energy using Einstein's mass-energy equivalence principle.
In this nuclear reaction, an alpha particle (He⁴) collides with Au¹⁹⁷ (gold) and produces Au¹⁹⁷ and another alpha particle.
To calculate the q value, we need to find the mass difference between the reactants and the products. The q value represents the energy released or absorbed during the reaction.
The reaction equation shows that the mass of the reactants (He⁴ + Au^197) is equal to the mass of the products (Au¹⁹⁷ + He⁴). Therefore, the mass difference is zero.
According to Einstein's mass-energy equivalence principle, E=mc^2, where E is the energy, m is the mass, and c is the speed of light.
Since the mass difference is zero, it means no energy is released or absorbed during this reaction. Hence, the q value is zero.
Calculations:
Mass of He⁴ = 4 atomic mass units (amu)
Mass of Au¹⁹⁷ = 197 atomic mass units (amu)
q value = (Mass of products) - (Mass of reactants)
= (197 amu + 4 amu) - (197 amu + 4 amu)
= 201 amu - 201 amu
= 0 amu
Therefore, the q value for this nuclear reaction is zero, indicating no energy release or absorption.
To learn more about nuclear reaction, here
https://brainly.com/question/13315150
#SPJ4
which if the following ionic compounds has the smallest lattice energy (the lattice energy least favorable to a stable lattice)? group of answer choices mgo bao csi lif
Answers
The smaller the ions are, the higher the lattice energy is, and vice versa. Similarly, the greater the charges on the ions, the higher the lattice energy is. Magnesium oxide (MgO), barium oxide (BaO), cesium iodide (CsI), and lithium fluoride (LiF) are all ionic compounds.
The lattice energy of an ionic compound is the energy required to break down the entire crystal into gaseous ions. Lattice energy depends on several factors, including the charges on the ions, their relative sizes, and their arrangements in the crystal lattice. The ionic compound with the smallest lattice energy, or the least favorable lattice energy for a stable lattice, is the one with the smallest ions and the lowest charges.
As a result, lithium fluoride (LiF) has the smallest lattice energy among the given options. LiF has the smallest ions (Li+ and F-) and the lowest charges (+1 and -1), which implies that its lattice energy is the lowest among the given options. As a result, LiF has the least unfavorable lattice energy for a stable lattice. Thus, the right answer is LiF.
To know more about compounds visit:
https://brainly.com/question/14117795
#SPJ11
HELP PLEASE
1. Locations where volcanoes form far from plate boundaries are called
A. islands
B. hot spots
C. divergent zones
Answers
Suppose a solid object is fully immersed in a liquid, but B is larger than w. Is this possible at all
Answers
This problem is stating a situation in which a solid object is fully immersed in a liquid and the buoyant force is larger that its weight, so that you need to discuss whether this is possible or not.
In general terms, it is necessary to keep in mind that when the buoyant force is larger than its weight, the object will float. On the other side, when the buoyant force is smaller than the weight, then the object will sink.
It means that it could be possible to have this scenario under specific conditions. Now, the Archimedes' principle can be applied with the following version:
\(F_B+W>m_{obj}*a\\\\\rho _{fluid}*V*g+m_{obj}*g>m_{obj}*a\\\\\rho _{fluid}*V-m_{obj}>m_{obj}*a\)
It means that the object can move down the liquid if has a significant acceleration (could be external), even when the buoyant force is larger than the weight
Learn more:
https://brainly.com/question/18103369https://brainly.com/question/14271593The gas-phase reaction between nitrogen and oxygen was carried out in a device designed to maintain constant pressure. There are two cylinders of equal volume with a reaction arrow between them. The cylinder on the left has two molecules of O 2 and two molecules of N 2. The cylinder on the right has four molecules of N O. A constant pressure is applied to both cylinders Write the balanced chemical equation for the reaction between nitrogen oxygen. Include physical states. Predict wether the work for the reaction is positive or negative or zero. Using the date determine the enthalpy of the reaction for the formation of 1 mole


Answers
Answer:
The answer should be that a constant pressure is applied to both cylinders and they are balanced chemical equations for the reaction between nitrogen oxygen.
Explanation:
If 33.9 ml of h3po4 neutralizes 23.4 ml of 2.28 m koh, what is the molarity of the phosphoric acid?
Answers
Answer: 1.57 M
Explanation:
\(M_{A}V_{A}=M_{B}V_{B}\\(33.9)M_{A}-=(23.4)(2.28)\\M_{A}=\frac{(23.4)(2.28)}{33.9}=\boxed{1.57 \text{ M}}\)

1) LIOH + HBr à LIBr + H2O
If you start with 5mol of lithium hydroxide, how many moles of lithium
bromide will be produced?
Answers
If Jill has a 2.9L of gas at a pressure of 5.5 atm and a temperature of 50˚C. What will be the temperature of the gas if I decrease the volume of the gas to 2.4L and decrease the pressure to 3.3 atm? Show work please!
Answers
The final temperature of the gas is determined as -112.6 ˚C.
What is the final temperature of the gas?
The final temperature of the gas is calculated by applying general gas equation as shown below;
V1P1/T1 = V2P2/T2
where;
V1 is the initial volume of the gasV2 is the final volume of the gasP1 is the initial pressure of the gasP2 is the final pressure of the gasT1 is the initial temperature of the gas = 50˚C = 50 + 273K = 323 KT2 is the final temperature of the gas = ?Substitute the given parameters and solve for the final temperature of the gas.
T2 = (V2P2T1)/(V1P1)
T2 = (2.4 x 3.3 x 323)/(2.9 x 5.5)
T2 = 160.38 K
In degrees Celsius = 160.38 - 273 = -112.6 ˚C
Thus, the final temperature of the gas is determined as -112.6 ˚C.
Learn more about general gas equation here: https://brainly.com/question/4147359
#SPJ1
60 POINTS PLEASE HELP
What is the average atomic mass for element z
Answers
2. Which equation is correctly balanced?
A. H₂+O₂ → H₂O
B.
Ca + Cl₂ → CaCl
C.
2H2 + O2 → 2H₂O
D.
Ca + Cl₂ → Ca₂Cl
Answers
The equation is correctly balanced is option C is correct 2H₂ + O₂ → 2H₂O
A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation
Here in the given reaction option C is correct because 4 atoms of hydrogen on reactant side. 2 atoms of oxygen on reactant side
2H₂ + O₂ → 2H₂O
And the other reaction is not balanced reaction because in reactant side or in product side there is difference so it is not balanced
Know more about balanced equation
https://brainly.com/question/12192253
#SPJ1
in the titration of naoh with vinegar, a student forgot to condition the buret, and it was wet with deionized water before filling with naoh solution. how will this affect the calculate molarity of naoh?
Answers
This NaOH calculation will have an impact on the molarity since there is a little quantity of deionized water present, which reduces the molarity of the NaOH, and because conditioning Bury will increase the volume needed to neutralize the acid.
The amount of NaOH added has an inverse relationship with its molarity. As a result, (CM)NaOH will decrease as NaOH increases. The moles of solute per liter of solution is measured as molarity. Molarity is represented by a capital M.
A solution is a combination of two or more compounds in chemistry in which neither ingredient undergoes chemical change. For instance, salt water is a mixture of salt and water (the solvent) (the solute). The amount of dissolved material in a solution is known as concentration.
To learn more about molarity
https://brainly.com/question/8732513
#SPJ4
1. which of the following elements is NOT multivalent?
A. Be
B. Cu
C. Pb
D. Fe
Answers
Answer: it is A .
hope this helps!!!!
Explanation:Be is not multivalent
What mass of iron should be produced if 11. 0g of aluminum react with 30. 0g of iron (III) oxide?
Answers
The mass of iron should be produced if 11. 0g of aluminum reacts with 30. 0g of iron (III) oxide is 10.50 g.
To determine the mass of iron produced, we need to use stoichiometry and the balanced chemical equation for the reaction between aluminum and iron(III) oxide.
The balanced chemical equation is:
2 Al + \(Fe_{2} O_{3}\) → + 2 Fe
From the equation, we can see that 2 moles of aluminum react with 1 mole of iron(III) oxide to produce 1 mole of iron.
First, we need to determine the limiting reactant by comparing the number of moles of aluminum and iron(III) oxide.
Moles of aluminum = mass of aluminum / molar mass of aluminum
= 11.0 g / 26.98 g/mol (molar mass of aluminum)
= 0.407 mol
Moles of iron(III) oxide = mass of iron(III) oxide / molar mass of iron(III) oxide
= 30.0 g / 159.69 g/mol (molar mass of iron(III) oxide)
= 0.188 mol
Since the stoichiometric ratio of aluminum to iron(III) oxide is 2:1, we can see that 0.188 mol of iron(III) oxide requires 0.376 mol of aluminum. However, we have only 0.407 mol of aluminum, which is in excess.
Therefore, the limiting reactant is iron(III) oxide. The amount of iron produced is determined by the moles of iron(III) oxide used. Moles of iron = 0.188 mol (same as moles of iron(III) oxide)
Now we can calculate the mass of iron produced using its molar mass (55.85 g/mol):
Mass of iron = Moles of iron × Molar mass of iron
= 0.188 mol × 55.85 g/mol
= 10.50 g
Therefore, the mass of iron produced is approximately 10.50 grams.
Know more about the Balanced chemical equation here:
https://brainly.com/question/13451900
#SPJ8
en un recipiente de 5l se introduce un gas oxígeno de 4 atm ¿ que presion ejercera si duplicamos el volumen del recipiente sin que varíe sutemperatura?
Answers
Answer:
Density: if you take alcohol and look at its density and divide the alcohol into two containers the density will be the same.
Temperature: if you take an ice cream and split it, the temperature will be the same in the two pieces.
Explanation:
If a sample of a gas occupies 5.22 L at 235 oC, what will its volume be at 75 oC if the pressure remains the same?
Answers
Answer:
1618.2
Explanation:
235+75=310×75=1618.2
Choosing location in supply chain is
1) one of the main strategic decisions
2) Depending on demand & supply forecasting
3) Aggregate planning & inventory management
4) All of the above.
Answers
Choosing location in supply chain is 4) All of the above.
Choosing location in the supply chain is one of the main strategic decisions that organizations have to make, itt depends on various factors such as demand and supply forecasting, aggregate planning, and inventory management. An appropriate location enables the firms to provide quality goods and services at the right time and place. The location strategy should consider several factors such as proximity to suppliers, customers, labor availability, transportation, etc.The location decision is important for the success of a business as it affects the cost of production, customer service, and market responsiveness. When a location is chosen, it affects the transportation and inventory cost.
Therefore, firms should consider the tradeoffs between the inventory carrying cost and the transportation cost when choosing a location. For instance, a firm may decide to locate closer to the customers to reduce the transportation cost but will have to incur higher inventory carrying cost due to the storage facilities. Hence, organizations need to carefully choose the best location in the supply chain that meets the needs of their customers and ensures their long-term success. Hence, the correct option is 4) All of the above.
Learn more about supply chain at:
https://brainly.com/question/31978808
#SPJ11
Identify the principle of separation.
i. raw rice and lentils
ii. sulphur and iron filings
ii. sand and sawdust
iv. oil and water
v. ammonium chloride, salt and sand
Answers
Explanation:
I. husking
ii. use magnet
III. husking
iv. floatation
v. Fractional distillation
A group of students wants to study the forces acting on a falling object. One of the students drops a tennis ball from rest at a second story balcony. As the tennis ball falls through the air, the air resistance acting on the ball is not zero. The students draw vectors to represent the forces acting on the ball while it is falling and speeding up.Which of the following vector representations best describes the forces acting on the falling ball and the net force on the ball?
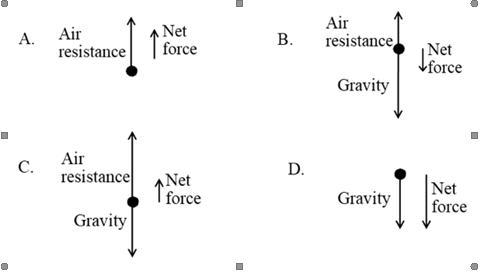
Answers
the answer is a Explanation:
The net force is in the direction of the force of gravity hence the correct answer is option B.
When an object falls freely within the gravitational field of the earth, the body experiences an acceleration known as the acceleration due to gravity which acts downwards. This is due to the force of gravity acting on the object.
The direction of the air resistance is opposite the direction of the force of gravity acting on the object. The net force is in the direction of the force of gravity hence the correct answer is option B.
Learn more about force of gravity: https://brainly.com/question/1479537
An unknown substance is a white solid at room temperature and has a melting point of 78 "c. Which of the following substances is most likely to be the identity of
the unknown sample?
Answers
The molecular solid naphthalene had always been the unidentified solid with such a melting point around 78 degrees Celsius.
At normal temperature as well as pressure, an ionic compound would be almost certainly a solid, even though a covalent compound could be a solid, a liquid, or even a gas.
The temperature when a solid can transform into a liquid is known as that of the melting point.
Strong intermolecular interactions have led to greater melting points for some substances, while weakly attractive molecules have lower melting points.
Learn more about melting point, here:
https://brainly.com/question/12826222
#SPJ1