Find a periodic table and look for the atomic mass of sodium, Na. What is it?
Answers
Answer:
Atomic mass: 22.989769 u
Explanation:
Related Questions
The surface currents of Earth’s oceans are most likely created by which of the following?
Answers
Answer:
global wind patterns. the rotation of the Earth. the shape of the ocean basins.
Explanation:
sample was analyzed and was found to contain 12.12% of carbon 16.17% of oxygen 71.71% of chlorine What is its empirical formula
Answers
To determine the empirical formula of the sample based on the given percentages of carbon, oxygen, and chlorine, we need to convert the percentages into moles and find the simplest whole number ratio between the elements.
Convert the percentages to moles:
Assume we have 100 grams of the sample.
Carbon (C): 12.12 grams (12.12% of 100g)
Oxygen (O): 16.17 grams (16.17% of 100g)
Chlorine (Cl): 71.71 grams (71.71% of 100g)
Convert the grams to moles using the respective atomic masses:
Carbon (C): Atomic mass of carbon = 12.01 g/mol
Moles of carbon = 12.12 g / 12.01 g/mol = 1.009 moles
Oxygen (O): Atomic mass of oxygen = 16.00 g/mol
Moles of oxygen = 16.17 g / 16.00 g/mol = 1.010 moles
Chlorine (Cl): Atomic mass of chlorine = 35.45 g/mol
Moles of chlorine = 71.71 g / 35.45 g/mol = 2.024 moles
Find the simplest whole number ratio:
To determine the simplest whole number ratio, divide the number of moles of each element by the smallest number of moles:
Carbon: 1.009 moles / 1.009 moles = 1
Oxygen: 1.010 moles / 1.009 moles = 1
Chlorine: 2.024 moles / 1.009 moles = 2
The simplest whole number ratio is 1:1:2, indicating that the empirical formula of the sample is C1O1Cl2.
However, it is important to note that the empirical formula represents the relative ratio of atoms in the compound and does not provide information about the actual molecular formula or the arrangement of atoms within the molecule. Further analysis, such as molecular weight determination, is necessary to determine the molecular formula.
Learn more about empirical formula Visit : brainly.com/question/1603500
#SPJ11
Carbon is classified as which type of element?
Answers
Carbon is classified as a nonmetal.
Which is amphoteric but not amphiprotic?Al2O3HCO3 -H2OHS-
Answers
Al2O3, or aluminum oxide, is an example of a compound that is amphoteric but not amphiprotic. Amphoteric substances have the ability to act as both an acid and a base, depending on the environment they are in. In the case of Al2O3, it can react with both acids and bases, forming salts and water. When reacting with an acid, it behaves as a base, and when reacting with a base, it behaves as an acid.
Amphiprotic substances, on the other hand, are a specific type of amphoteric compounds that can donate and accept a proton (H+ ion) in their reactions. Amphiprotic substances are always amphoteric, but not all amphoteric substances are amphiprotic.
Al2O3 is not amphiprotic because it does not have any protons to donate or accept in its reactions. The other compounds listed, HCO3- (hydrogen carbonate), H2O (water), and HS- (hydrogen sulfide ion), are all examples of amphiprotic substances. They can each donate and accept a proton in their reactions, making them both amphoteric and amphiprotic.
In summary, Al2O3 is an amphoteric substance due to its ability to react with both acids and bases, but it is not amphiprotic as it does not involve proton transfer in its reactions. The other listed compounds, HCO3-, H2O, and HS-, are examples of amphiprotic substances that exhibit both amphoteric and amphiprotic behavior.
To know more Al2O3HCO3 -H2OHS- click this link-
brainly.in/question/22983203
#SPJ11
which substance will dissolve in water to produce an acidic solution? (a) fecl3 (b) na2o (c) nac2h3o2 (d) nh3
Answers
The correct option is C, The substance that will dissolve in water to produce an acidic solution is \(nac_2h_3o_2\), also known as sodium acetate.
An acidic solution is a type of solution in chemistry that has a high concentration of hydrogen ions (H+) relative to hydroxide ions (OH-). In an acidic solution, the pH level is less than 7 on a scale of 0-14, with 0 being the most acidic and 14 being the most basic. Acids are substances that donate hydrogen ions to a solution, which can then react with other substances to form chemical bonds.
Acidic solutions have several distinct properties, including a sour taste, the ability to react with metals to produce hydrogen gas, and the ability to turn litmus paper red. Strong acids, such as hydrochloric acid (HCl) and sulfuric acid (\(H_2SO_4\)), can be highly corrosive and can cause burns and other injuries if handled improperly.
To know more about Acidic solution refer to-
brainly.com/question/30549961
#SPJ4
which of the following compounds is the most reactive dienophile in a diels-alder reaction with 1,3-butadiene? ch3ch≡chch3 ch2= choch3 ch2= ch2 ch2= chcho (ch3)2c=ch2
Answers
The most reactive dienophile in a Diels-Alder reaction with 1,3-butadiene is CH2=CHCHO (acrolein)
What Is Diels-Alder reaction? Analyzing The Given Compounds.The Diels-Alder reaction is a chemical reaction that combines a conjugated diene and a dienophile to form a cyclic compound.
The reactivity of a dienophile is determined by its ability to accept electron density and undergo the necessary bond-forming process.
Among the given options, CH2=CHCHO (acrolein) is the most reactive dienophile in a Diels-Alder reaction with 1,3-butadiene. This is because acrolein contains an electron-withdrawing carbonyl group (C=O) attached to an alkene (CH2=CH).
The electron-withdrawing nature of the carbonyl group increases the electrophilic character of the alkene, making it more susceptible to nucleophilic attack by the electron-rich diene, 1,3-butadiene.
The other compounds in the options (CH3CH≡CHCH3, CH2=CHOCH3, CH2=CH2, and (CH3)2C=CH2) lack the electron-withdrawing carbonyl group, reducing their reactivity as dienophiles in a Diels-Alder reaction with 1,3-butadiene.
Learn more about Diels-Alder reaction
brainly.com/question/30751490
#SPJ11
If 8. 0 mg of a radioactive substance naturally decays to 0. 50 mg over 184 days, what is the half-life of the radioisotope?.
Answers
The half-life of radioactive elements is 46 days.
We need to know about the half-life of the radioactive elements to solve this problem. The radioactive element will decay over time depends on the half-life and follow the equation
N = No(1/2)^(t/t'')
where N is the final quantity, No is the initial quantity, λ is the decaying constant, t is time and t'' is the half-life of a radioactive element.
From the question above, we know that
No = 8 mg
N = 0.5 mg
t = 184 days
By substituting the given parameters, we can calculate the half-life
N = No . (1/2)^(t / t'')
0.5 = 8(1/2)^(184 / t'')
1/16 = (1/2)^(184 / t'')
(1/2)^4 = (1/2)^(184 / t'')
4 = 184/t''
t'' = 184/4
t'' = 46 days
Find more on half-life at: https://brainly.com/question/25750315
#SPJ4
How much space does 2 x 10^22 atoms of copper take up?
Answers
Answer:
2.35mL
Explanation:
The amount of space a substance holds is its volume.
Density = mass (g) /volume (L)
Where;
mole = mass/molar mass
Since 1 mol of Cu contains 6.022 × 10^22 atoms.
There are 2 x 10^22/6.022 × 10^22 moles in 2 x 10^22 atoms of copper
2 x 10^22/6.022 × 10^22
2/6.022 × 10^(22-22)
0.332 × 10^0
0.332mol
Mole = mass/molar mass
0.332 = mass/63.5
mass = 63.5 × 0.332
mass = 21.08g
Density = mass/volume
Density of copper (Cu) is 8.96 g/cm³
8.96 = 21.08/v
V = 21.08/8.96
V = 2.35mL or cm³
Which electron dot diagram shows how hydrogen and oxygen are bonded together in the compound H2O?
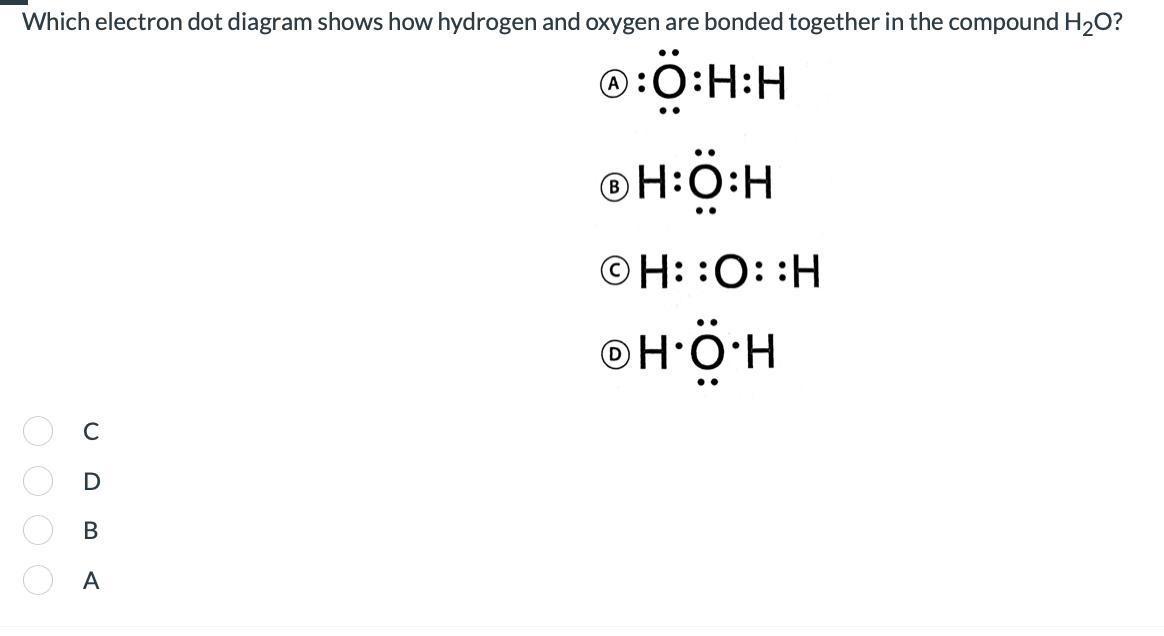
Answers
The correct electron dot structure of water is shown by option B
What is electron dot structure?Each valence electron in the electron dot structure is represented by a dot that is positioned around the element's atomic symbol.
The Lewis dot structure, sometimes referred to as the electron dot structure, uses dots to represent the valence electrons of an atom.
Water contains two hydrogen and one oxygen atom with the oxygen atom having two lone pairs of electrons as shown above in the question.
Learn more about electron dot structure:https://brainly.com/question/9840379
#SPJ1
In forming an alkyne by elimination from a dihalide, it is ______ difficult to remove the second equivalent of HX to form an alkyne because sp2 hybridized carbons have ______ and ______ bonds to the H and X after the first elimination.
Answers
The sp2 hybridized carbons have shorter and stronger bonds than the sp3 hybridized carbon atoms.
What is a dihalide?A dihalide is an organic compound that contains two halogen atoms that are located on different carbon atoms hence they can be eliminated during dehydrohalogenation reactions.
We must note that it is more difficult to form an alkyne from a dihalide because the sp2 hybridized carbons have shorter and stronger bonds than the sp3 hybridized carbon atoms.
Learn more about sp2 hybridized carbons: https://brainly.com/question/6920150
Which of the following types of mass movement is LEAST coherent (most like a fluid)?
a. slump
c. rock slide
b. creep
d. mudflow
Answers
The type of mass movement that is LEAST coherent (most like a fluid) is a mudflow. The correct option is d.
Mass movement refers to the downhill movement of earth materials due to gravity. There are different types of mass movement, including slump, rockslide, creep, and mudflow. The coherency of a mass movement refers to the degree of internal strength or viscosity of the material involved.
The more coherent the material, the less it flows like a fluid. Among the given options, mudflow is the least coherent or most fluid-like type of mass movement. Mudflow refers to the rapid downhill movement of a mixture of water and fine-grained sediment, such as clay and silt.
Mudflows are highly fluid and can travel at high speeds, posing a significant hazard to life and property in areas prone to landslides and flash floods. In contrast, slumps, rockslides, and creep involve more cohesive materials and exhibit less fluid-like behavior. Therefore, the correct option is d.
To know more about mudflow refer here:
https://brainly.com/question/30922995#
#SPJ11
A gas mixture contains 1.52 atm of Ne, 766 mmHg of He and Ar. What is the partial pressure, in atmospheres, of At if the gas mixture has a total pressure of 3.27atm
Answers
Answer:
0.74 atm.
Explanation:
From the question given above, the following data were obtained:
Pressure of Ne (Pₙₑ) = 1.52 atm
Pressure of He (Pₕₑ) = 766 mmHg
Total pressure (Pₜ) = 3.27 atm
Pressure of Ar (Pₐᵣ) =?
Next, we shall convert the pressure of He from mmHg to atm. This can be obtained as follow:
760 mmHg = 1 atm
Therefore,
766 mmHg = 766 mmHg × 1 atm / 760 mmHg
766 mmHg = 1.01 atm
Finally, we shall determine the partial pressure of Ar. This can be obtained as follow:
Pressure of Ne (Pₙₑ) = 1.52 atm
Pressure of He (Pₕₑ) = 1.01 atm
Total pressure (Pₜ) = 3.27 atm
Pressure of Ar (Pₐᵣ) =?
Pₜ = Pₙₑ + Pₕₑ + Pₐᵣ
3.27 = 1.52 + 1.01 + Pₐᵣ
3.27 = 2.53 + Pₐᵣ
Collect like terms
3.27 – 2.53 = Pₐᵣ
Pₐᵣ = 0.74 atm
Thus the partial pressure of Ar is 0.74 atm.
Hippos spend most of their time in rivers, but they come out of the water to eat grass. Right now, it's the middle of the night. The sun is not shining, and the hippos are not eating. What is happening to the carbon in the air around the hippos and the grass nearby?
Answers
Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 93.4 g/mL times 20. mL = g 489.7 nm 53.061 s = m/s 216.3 m 66.4 s = m/s
Answers
For the first calculation, 93.4 g/mL times 20 mL = g, the answer is 1868 g. The original measurements contained four and three significant digits, respectively, so the answer should have three significant digits (1868 g). For the second calculation, 489.7 nm times 53.061 s = m/s, the answer is 2609.74731 m/s. The original measurements contained four and five significant digits, respectively, so the answer should have five significant digits (2609.74731 m/s). For the third calculation, 216.3 multiplied 66.4 s = m/s, the answer is 14393.2 m/s.
The original measurements contained three and two significant digits, respectively, so the answer should have three significant digits (14393.2 m/s). To multiply or divide the following measurements, it is important to consider the number of significant digits of each measurement. To multiply or divide with accuracy, you should use the fewest number of significant digits in your final answer.
Know more about significant digits here:
https://brainly.com/question/1658998
#SPJ11
the central ray of the beam in most pantomographic units is aimed (a) upwards, (b) horizontally (c) downwards
Answers
Option B: In most pantomographic units, the central ray of the X-ray beam is directed horizontally.
By aiming the central ray horizontally, the X-ray machine can rotate around the patient's head in a semi-circular motion. During this rotation, the X-ray detector and the X-ray source move simultaneously in opposite directions. This synchronized movement allows for a continuous exposure of the X-ray film or sensor, creating a panoramic image.
The horizontal positioning of the central ray enables the panoramic X-ray machine to capture a wide field of view that includes both the upper and lower jaws, teeth, surrounding bone structures, and other important anatomical features. This comprehensive image assists dental professionals in evaluating the overall dental and skeletal structures, identifying dental abnormalities, assessing impacted teeth, examining the temporomandibular joint, and detecting potential pathology.
To know more about X-ray, refer:
https://brainly.com/question/31170943
#SPJ4
why does nitrogens lewis structure have 5 dots around it while nitrogens Bohr diagram has seven dots around it?
Answers
Answer:
Bohr diagram shows electrons orbiting the nucleus. Nitrogen has 7 electrons orbiting the nucleus
Lewis structure is the simplified Bohr diagram. It only shows the electrons in the outer shell. For Nitrogen, 2 electrons are in the first shell. The remaining 5 electrons are in the outer shell.
Explanation:
Bohr diagram shows electrons orbiting the nucleus. Nitrogen has 7 electrons orbiting the nucleus
Lewis structure is the simplified Bohr diagram. It only shows the electrons in the outer shell. For Nitrogen, 2 electrons are in the first shell. The remaining 5 electrons are in the outer shell.
Why is it important for scientists to publish a
description of their procedures along with the results
of their experiments?
Answers
Answer:
Anything related to science needs to be detailed and understood by a range of people. Or, what they published may not create any impact if you can't read it and understand what you just read.
Explanation:
one liter of cacl2 solution contains 2.5 moles of cacl2. what is the molarity of th solution
Answers
The molarity of the CaCl₂ solution, which contains 2.5 moles of CaCl₂ in one liter, is 2.5 mol/L.
Molarity is a measure of the concentration of a solute in a solution, expressed as the number of moles of solute per liter of solution (mol/L). In this case, the given information states that one liter of the CaCl₂ solution contains 2.5 moles of CaCl₂.
To calculate the molarity, we divide the number of moles of solute (CaCl₂) by the volume of the solution in liters (1 L):
Molarity = Number of moles of solute / Volume of solution (in liters)
Molarity = 2.5 moles / 1 L
Molarity = 2.5 mol/L
learn more about molarity here:
https://brainly.com/question/8732513
#SPJ4
Make a prediction about why it has been useful for chemists and physicists to categorize elements on the periodic table of elements based on similar attributes.
Answers
The elements are categorized based on similar attributes so that it could be easier to collectively study the properties of the elements.
What is the periodic table?The periodic table is arrangement of the elements based on the atomic number of the elements. This implies that the elements are arranged in the order of increasing atomic numbers of the elements. The atomic numbers of the elements therefore tend to increase from left to right. This is how the Mendeleev table of elements was arranged.
Now, let us know that the periodic table is arranged in groups and periods. The elements in the same group have similar chemical properties while the elements in the same period only have the same number of shells or the same highest energy level.
Having said this, the elements are categorized based on similar attributes so that it could be easier to collectively study the properties of the elements.
Learn more about the periodic table:https://brainly.com/question/11155928
#SPJ1
Describe Lavoisier's famous presentation and why his assertion of
L.O.C.O.M. replaced the Phlogiston Theory of ordinary burning. In this
assignment you must briefly research the Phlogiston Theory of ordinary
burning.
Answers
The Phlogiston Theory of ordinary burning posited that the materials that were combustible all contained a material called Phlogiston.
What is the Phlogiston Theory of ordinary burning?
In science, theories are often put forward to explain a phenomenon. theory may not survive the test of time when contrary evidences begin to emerge.
The Phlogiston Theory of ordinary burning posited that the materials that were combustible all contained a material called Phlogiston which is released when the material is burnt.
However, Lavoisier was able to show by experiment that this position was a mere speculation and the the combustion of a substance was due to its reaction with oxygen.
Learn more about Phlogiston Theory:https://brainly.com/question/13070283
#SPJ1
Which of these civilizations still has strong cultural and religious ties to their star
knowledge?
A. Greece
B. Western Europe
C. Egypt
D. D/Lakota
Answers
Answer:
In the 2nd millennium, the eastern coastlines of the Mediterranean are dominated by the Hittite and Egyptian empires, competing for control over the city states in the Levant (Canaan)
Explanation:
because it is
2 NH3 (g)+ H2SO4 -> (NH4)2SO4 (s)
1. If 225 kg of ammonium sulfate is to be made in one batch, how many liters of ammonia at STP are needed?
2. How many moles of H2SO4 are required?
3. If the H2SO4 is in the form of a 6.00 M solution, what volume of this solution is needed (provide your answer in liters)?
Answers
we need 283.6 liters of the 6.00 M H2SO4 solution to react with the NH3 and produce 225 kg of (NH4)2SO4.
The balanced equation tells us that 2 moles of NH3 reacts with 1 mole of H2SO4 to produce 1 mole of (NH4)2SO4.
Using the molar mass of (NH4)2SO4 (132.14 g/mol), we can calculate the number of moles required to make 225 kg of (NH4)2SO4:
225 kg / 132.14 g/mol = 1701.6 mol (NH4)2SO4
Therefore, we need half as many moles of NH3:
1701.6 mol / 2 = 850.8 mol NH3
At STP (standard temperature and pressure), 1 mole of any ideal gas occupies 22.4 liters. Therefore, the required volume of ammonia at STP is:
850.8 mol NH3 x 22.4 L/mol = 19,069.92 L NH3
From the balanced equation, we can see that the stoichiometric ratio between H2SO4 and (NH4)2SO4 is 1:1. Therefore, we need the same number of moles of H2SO4 as we calculated for (NH4)2SO4 in part 1:
1701.6 mol H2SO4
To determine the volume of the 6.00 M H2SO4 solution required, we need to use the molarity equation:
Molarity = moles of solute / liters of solution
Rearranging this equation gives:
Liters of solution = moles of solute / molarity
Substituting the values we have:
Liters of solution = 1701.6 mol / 6.00 mol/L = 283.6 L
Therefore, we need 283.6 liters of the 6.00 M H2SO4 solution to react with the NH3 and produce 225 kg of (NH4)2SO4.
To know more about molarity, visit:
https://brainly.com/question/8732513
#SPJ1
A chemist mixes 50.0mL of a 1.0M NaOH solution with 50.0mL of a 1.0M Ba(OH)2 solution. Assuming the two solutions are additive, what is the pH of the resulting solution
Answers
Answer:
\(pH=14.2\)
Explanation:
Hello there!
In this case, according to the information in this problem, and considering these two bases are strong, it is necessary for us to calculate the total moles of OH ions as shown below:
\(n_{OH^-}^{from\ NaOH}=0.050L*1.0mol/L=0.050mol\\\\n_{OH^-}^{from\ Ba(OH)_2}=0.050L*1.0mol/L*2=0.10mol\\\\n_{OH^-}^{tot}=0.15mol\)
Now, the as the solutions are additive, the total volume is then 0.100 L and the concentration:
\([OH^-]=\frac{0.15mol}{0.100L}=1.5\)
And therefore, the pH is:
\(pH=14+log(1.5)\\\\pH=14.2\)
Regards!
a balloon holds a certain volume of air. in what three ways can the volume of the balloon be increased?•_____the number of air molecules in the balloon •_____the temperature of the balloon •_____the pressure on the balloon increasing/decreasing for all blanks
Answers
wThe question requires us to define the ways the volume of a baloon can be increased, considering number of molecules of air, temperature and pressure.
To answer this question, we must analyze the ideal gas law, which can be written as:
\(P\times V=n\times R\times T\)where P is the pressure of the system, V is its volume, n is the number of moles, R is a constant (constant of gases) and T refers to the temperature of the system.
We can rearrange this equation to analyze the effect of P, n and T over V:
\(V=\frac{n\times R\times T}{P}\)Looking at the equation, we can note that temperature (T) and number of moles (n) are directly proportional to volume (V), while pressure (P) is inversely proportional.
This means that increasing / decreasing the number of moles or the temperature of the system will increase / decrease the volume, while increasing the pressure will decrease the volume (and decreasing the pressure will increase volume):
\(\begin{gathered} \uparrow T=\uparrow V\text{ and }\downarrow T=\downarrow V \\ \uparrow n=\uparrow V\text{ and }\downarrow n=\downarrow V \\ \uparrow P=\downarrow V\text{ and }\downarrow P=\uparrow V \end{gathered}\)Considering the information above, we can answer the question as it follows:
• increasing the number of air molecules in the balloon
• increasing the temperature of the balloon
• decreasing the pressure on the balloon
configurate the following element using sub level and group each elemnt v(z=23),Ni(z=28),Cu(z=29),zn(z=30),cr(z=24),Mn(z=25)
Answers
Answer:
To configure the electron sublevels and groups for each element, we can use the following format:
Element symbol: [Electron configuration] Sublevel: Group
V (Z=23): [Ar] 3d3 4s2 Sublevel: 3d, 4s Group: 5, 4
Ni (Z=28): [Ar] 3d8 4s2 Sublevel: 3d, 4s Group: 10, 4
Cu (Z=29): [Ar] 3d10 4s1 Sublevel: 3d, 4s Group: 11, 4
Zn (Z=30): [Ar] 3d10 4s2 Sublevel: 3d, 4s Group: 12, 4
Cr (Z=24): [Ar] 3d5 4s1 Sublevel: 3d, 4s Group: 6, 4
Mn (Z=25): [Ar] 3d5 4s2 Sublevel: 3d, 4s Group: 7, 4
Number 1 I need help please

Answers
Answer:
a. The chemical symbols represent the chemical element and are 1 to 2 letters long.
Example: Er- Erbium
b. The chemical formulas identify each element by it's chemical symbol and indicates the proportionate number of atoms in each element.
Example: Salt- NaCI
c. A chemical equation is the symbolic equation of a chemical reaction in the form of symbols and formulas.
Example: H2+O=H2O (One water molecule)
Construct an explanation for why convection in orbit around Earthfor example, aboard the International Space Station --would be impossible .
Answers
The characteristics of the atmospheric pressure allow to find the reasons for the heat transfer processes.
Convection does not occur due to the very small outside pressure, which implies that there is no mass of gases that can move
Thermal conduction is the process by which bodies exchange thermal energy (heat), these processes can be divided:
Conduction. It is when the body is in contact and thermal energy is exchanged from the hottest to the coldest body, without movement of matter. Convection. It occurs when for the exchange of energy there is a movement of matter Radiation. The emission of electromagnetic radiation in the energy exchange medium occurs in all bodies at more than 0K.
In space at the height of the space station 400 km, than the area called the thermostat or ionosphere, in it the atmospheric pressure is very low. There are ionized gases, nitrogen, oxygen and helium, but at very low pressure.
Because there are not enough gases around the space station, the convection processes that are due to the movement of a mass of gas do not exist.
At the height of the space station the most important mechanism of heat transfer is radiation.
In conclusion, the characteristics of atmospheric pressure allow us to find the reasons for the heat transfer processes.
Convection does not occur due to the very small outside pressure, which implies that there is no mass of gases that can move
Learn more about the thermal conduction process here:
https://brainly.com/question/862061
estions
ta10t04h_ch01.01m
Check My Work (2 remaining)
Ο Ο Ο Ο Ο
Jamal is a student in the health care professions class. He had a very exciting day at clinical and decided to post about
it on his social media account. He posted the following “Great day saving lives today! I did CPR and saved the patient!"|
Jamal did not post his location or the patient's personal health information; however, a friend of his (Dario) saw the
post and realized that his neighbor was in a similar situation that day, Dario called his neighbor's wife and inquired as
to his health. During the conversation, Dario told the wife that his friend was there and performed the CPR. The wife
reported Jamal to his program director,
Which law did Jamal violate?
Оа. НІРАА
b. FERPA
c. Affordable Care Act
d. None
Answers
Answer:
The correct option is;
a. HIPAA
Explanation:
The federal law which safeguards Personal Health Information (PHI) is the health Insurance Portability and Accountability (HIPAA) Act of 1996 with rules regarding the security, privacy and transmission (electronically) of medical health data, was passed to improve the ease healthcare movement between healthcare providers has also added new limitations on PHI disclosure
The HIPAA restricts with whom, when, how and where PHI can be disclosed including physically (direct in person or writing) or electronically (online, over the phone)
Therefore, Jamal had violated HIPAA.
safe laboratory practices should be followed by all chemists and other scientists true or false
Answers
Answer:
True
Explanation:
Answer:
True
Explanation:
how many grams of tin (ll) fluoride are produced if 45.0 grams HF are reacted
Answers
Approximately 176.3 grams of tin (II) fluoride (SnF2) are produced when 45.0 grams of HF react.
The balanced chemical equation for the reaction between hydrofluoric acid (HF) and tin (II) fluoride (SnF2) is:
2 HF + SnF2 → SnF4 + 2 HCl
This equation tells us that for every 2 moles of HF that react with SnF2, we will get 1 mole of SnF4 produced.
To determine how many grams of SnF2 are produced from 45.0 grams of HF, we need to first convert the mass of HF to moles using its molar mass. The molar mass of HF is approximately 20.01 g/mol:
45.0 g HF × (1 mol HF / 20.01 g HF) = 2.25 mol HF
According to the balanced equation, 2 moles of HF react with 1 mole of SnF2. Therefore, we can determine the moles of SnF2 produced by dividing the moles of HF by 2:
2.25 mol HF ÷ 2 = 1.125 mol SnF2
Finally, we can convert the moles of SnF2 to grams using its molar mass, which is approximately 156.70 g/mol:
1.125 mol SnF2 × (156.70 g SnF2 / 1 mol SnF2) ≈ 176.3 g SnF2
For more question on tin (II) fluoride click on
https://brainly.com/question/29715194
#SPJ11