Express the equilibrium constant for the following reaction.
16 CH3Cl(g) + 8 Cl2(g) ? 16 CH2Cl2(g) + 8 H2(g)
Question 1 options:
K=[CH3Cl2] [H2][CH3Cl] [Cl2]K=[CH3Cl2] [H2][CH3Cl] [Cl2] {"version":"1.1","math":"K=[CH3Cl2] [H2][CH3Cl] [Cl2]"}
K=[CH2Cl2]16 [H2]8[CH3Cl]16 [Cl2]8K=[CH2Cl2]16 [H2]8[CH3Cl]16 [Cl2]8 {"version":"1.1","math":"K=[CH2Cl2]16 [H2]8[CH3Cl]16 [Cl2]8"}
K=[CH3Cl]16 [Cl2]8[CH2Cl2]16 [H2]8K=[CH3Cl]16 [Cl2]8[CH2Cl2]16 [H2]8 {"version":"1.1","math":"K=[CH3Cl]16 [Cl2]8[CH2Cl2]16 [H2]8"}
K=[CH3Cl] [Cl2][CH2Cl2] [H2]K=[CH3Cl] [Cl2][CH2Cl2] [H2] {"version":"1.1","math":"K=[CH3Cl] [Cl2][CH2Cl2] [H2]"}
K=[CH3Cl]1/2 [Cl2][CH2Cl2]1/2 [H2]
Answers
The equilibrium constant for the reaction 16 \(CH^3Cl\)(g) + 8\(Cl^2\)(g) ⇌ 16 \(CH^2Cl^2\)(g) + 8 \(H^2\)(g) can be expressed as:
K = \([CH^2Cl^2]^{16} [H2]^8 / ([CH^3Cl]^{16} [Cl^2]^8)\)
In this expression, K represents the equilibrium constant, and the terms in brackets represent the concentrations of each species at equilibrium.
The equilibrium constant for a chemical reaction is defined as the ratio of the product concentrations raised to their stoichiometric coefficients over the reactant concentrations raised to their stoichiometric coefficients, each raised to a power equal to the coefficient. Mathematically, it can be expressed as:
K = ([products]^stoichiometric coefficients)/([reactants]^stoichiometric coefficients)
In the given reaction, the stoichiometric coefficients are 16, 8, 16, and 8 for \(CH^3Cl, Cl^2, CH^2Cl^2, and H^2,\) respectively. Therefore, the equilibrium constant expression is:
K = \([CH^2Cl^2]^{16} [H2]^8 / ([CH^3Cl]^{16} [Cl^2]^8)\)
This means that the equilibrium constant for the reaction is equal to the ratio of the concentrations of the products raised to their stoichiometric coefficients to the concentrations of the reactants raised to their stoichiometric coefficients, each raised to a power equal to the coefficient.
The value of the equilibrium constant depends on the temperature at which the reaction occurs and is a measure of the extent to which the reaction proceeds towards products or reactants at equilibrium.
To know more about "Equilibrium constant" refer here:
https://brainly.com/question/31313431#
#SPJ11
Related Questions
Use the model provided to select ALL of the examples of mixtures.
A) iced tea
B) salt water
C) table sugar
D) orange juice
E) aluminum oxide
Answers
Answer:
Explanation:
Ice tea
Salt water
Orange juice
Calculate the Theoretical Yield of your product, i.e. the mass you would expect to recover, assuming 100% conversion to product.(explain it too)
Answers
The Theoretical Yield of the reaction is 2g.
The theoretical yield is the amount of product expected to be recovered in a chemical reaction, assuming perfect conversion of all reactants to products.
To calculate the theoretical yield of your product, you need to know the mass of each of the reactants and the stoichiometry of the reaction.
Let's assume that the reaction is A + B → C. The theoretical yield is equal to the mass of reactant A multiplied by the stoichiometry of C, divided by the stoichiometry of A. The equation looks like this:
Theoretical Yield = (Mass of Reactant A) × (Stoichiometry of C) / (Stoichiometry of A)
For example, if you have 1 g of reactant A, the stoichiometry of C is 2, and the stoichiometry of A is 1, the theoretical yield would be 2 g.
Theoretical Yield = (1 g) × (2) / (1) = 2 g
To know more about Theoretical Yield click on below link:
https://brainly.com/question/14966377#
#SPJ11
Explain how acids and bases directly or indirectly affect the hydrogen ion concentration of a solution.
Answers
Based on the definition of Brønsted-Lowry acids and bases, hydrogen ion concentration increases at the dissociation of acid and decreases at the dissociation of a base.
There are various definitions of acids and bases according to different chemists such as Svante Arrhenius, Johannes Nicolaus Brønsted, and Thomas Martin Lowry.
An Arrhenius acid is a substance that ionizes in water and produces H+ ions while an Arrhenius base is a substance that ionizes in water and produces -OH ions.
On the other hand, Brønsted-Lowry defines an acid as a proton or hydrogen ion donor and a base as a proton or hydrogen ion acceptor. This definition of acids and bases is more suitable to answer your question. The dissociation of Brønsted-Lowry acids and bases is shown below.
Acid Dissociation: HA → \(H^{+} +A^{-}\)
Base Dissociation: B + H2O → \(HB^{+} + OH^{-}\)
The dissociation of Brønsted-Lowry acid increases the hydrogen ion concentration in the solution. In contrast, the Brønsted-Lowry base dissociates by taking the hydrogen ion to produce hydroxide ion, which means that the hydrogen ion concentration of the solution decreases.
For more information regarding acids and bases, please refer to the link https://brainly.com/question/6904846.
#SPJ4
This formula equation is unbalanced.
P4(s) + Cl2(g) Right arrow. PCl3(l)
Which coefficient should be placed in front of PCl3 to balance this equation?
1
2
4
6
Answers
Answer:
4
Explanation:
Answer to balance the equation
P4 + 6Cl2 => 4PCl3
Answer:
C
Explanation:
Which of the following is an example of how the same chemicals used to
help people can harm the environment?
OA. Hillsides erode after plants are removed to clear a space for
O B. Phosphates are removed from detergents so they don't end up in
c. Excess fertilizers run off into rivers and create a dead zone.
OD. Plants grow back after toxic chemicals are removed from the
crops.
drinking water.
surrounding soil
Answers
Answer:
c. Excess fertilizers run off into rivers and create a dead zone.
Explanation:
One very clear cut example of how the same chemicals used to help people can harm the environment is shows is through the excess fertilizers that run off into rivers and create a dead zone.
Fertilizers are chemical substances that are used to increase farm yield. They are rich in nutrients that plants would need to ensure their proper growth. When these fertilizers gets washed into water bodies, the cause enrichment and a nutrient glut. It takes a lot of energy and oxygen to break them down thereby leading to a dead zone formation.what are the 5 benefits of changing colour/paint of the
laboratories and auditoriums?
Answers
Answer:
AestheticsImproved Focus and ConcentrationStress ReductionPositive ImpressionIncreased CreativityExplanation:
why do water and solutes leave capillaries at the arterial end?
Answers
The water and the solutes leave the capillaries at the arterial end because the blood pressure is the greater than the osmotic pressure at the arterial end.
The blood pressure is the greater than the osmotic pressure at the arterial end and the water molecules and the solutes leave the capillaries at the arterial end. At the arterial end of capillary, the blood pressure is exceeds the limit of the the osmotic pressure, that will causes the net movement of the fluid out of the capillary.
Thus, due to the higher blood pressure than the osmotic pressure the solutes and the water leaves the artial end.
To learn more about arterial end here
https://brainly.com/question/12192676
#SPJ4
2Al + 6HCl → 2AlCl3 + 3H2
If the chemical reaction produces 129 grams of AlCl3, how many grams of H2 are also produced?
Answers
Answer: The reaction produces 2.93 g H₂.
M_r: 133.34 2.016
2Al + 6HCl → 2AlCl₃ + 3H₂
Moles of AlCl₃ = 129 g AlCl₃ × (1 mol AlCl₃/133.34 g AlCl₃) = 0.9675 mol AlCl₃
Moles of H₂ = 0.9675 mol AlCl₃ × (3 mol H₂/2 mol AlCl₃) = 1.451 mol H₂
Mass of H₂ = 1.451 mol H₂ × (2.016 g H₂/1 mol H₂) = 2.93 g H₂
Explanation:
If the initial metal sulfide precipitate is black with traces of yellow, what lon is likely to be present? o Tin(IV) Ion o Lead (ii) ion o Copper (ii) ion o Bistmuth (ii) lon
Answers
Copper (II) ion is likely to be present if the initial metal sulfide precipitate is black with traces of yellow.
Copper (II) sulfide is black in color, which matches the color of the initial precipitate. However, when exposed to air, copper (II) sulfide can partially oxidize to form copper (II) oxide, which is yellow in color. Therefore, traces of yellow in the precipitate indicate the presence of copper (II) ion. Tin (IV) ion, lead (II) ion, and bismuth (II) ion do not form black sulfides, and therefore cannot be the cause of the initial precipitate. Copper (II) ion is likely to be present if the initial metal sulfide precipitate is black with traces of yellow.
learn more about metal sulfide here:
https://brainly.com/question/14839148
#SPJ11
How many total atoms in 3CuSO4
Answers
Answer:
18
Explanation:
Element Symbol no of Atoms
Copper Cu 3
Sulfur S 3
Oxygen O 12
18 = total
To see the number of atoms of an element in a given molecule we need to multiply stoichiometry to the number that is written on the foot of the element that is stoichiometry. Therefore, there are total 18 electrons in the given compound.
What is atom?Atom is the smallest particle of any element, molecule or compound. Atom can not be further divided. Atoms contains nucleus in its center and electron that revolve around the atom in fixed orbit.
In the nucleus, proton and neutron are present. Electron has -1 charge while proton has +1 charge. Neutron is neutral that is it has no charge. So overall the charge of nucleus is due to only proton, not by neutron.
Element Symbol no of Atoms
Copper Cu 3
Sulfur S 3
Oxygen O 12
S, there are total 18 electrons in the given compound.
Therefore, there are total 18 electrons in the given compound.
To know more about atom, here:
https://brainly.com/question/13518322
#SPJ2
two of the readings above focus on stomach ph and its regulation. how is it possible for a human organ to be so acidic without damaging its own tissue and that of surrounding organs (be specific with regard to the chemistry of any destructive/protective processes)? what is a cause of an ailment that results from this acidic environment not being correctly regulated or contained? describe treatment options for this ailment, and explain how they address the imbalance from a chemical perspective.
Answers
Numerous mucosal defense mechanisms in the stomach guard it against toxic substances including hydrochloric acid. The mucus-bicarbonate barrier makes up the pre-epithelial defense. The pH gradient created by mucus and the bicarbonate that mucus cells release helps to keep the epithelial cell surface close to neutral.
Stomach acid or bile that irritates the lining of the food pipe can cause acid reflux, a digestive disorder.
When bile or stomach acid enters the food pipe and irritates the lining, the condition becomes chronic. More than twice a week episodes of heartburn and acid reflux can be signs of GERD.
Burning chest discomfort is one of the symptoms, which normally gets worse when you lie down after eating.
Some of the treatments for this ailment are:
Antacids, which can neutralize stomach acid, include Alka-Seltzer, Maalox, Mylanta, Rolaids, and Riopan, as possible treatments for this condition. If antacids are ineffective, your doctor might consider one of the following drugs:
Your stomach is coated with foaming agents (Gaviscon) to stop reflux.Pepcid and Tagamet are H2 blockers that reduce acid production.Aciphex, Nexium, Prilosec, Prevacid, and Protonix are examples of proton pump inhibitors that also lessen stomach acid production.The LES can be strengthened using prokinetics (Reglan, urecholine), which can also speed up stomach emptying and lessen acid reflux.Visit the link below to learn more about Stomach acids:
brainly.com/question/28301853
#SPJ4
Which of the following summaries expresses the main points of the passage best?
I believe gravity is the most important aspect of our universe. Without it we would all be floating off
into the universe. There wouldn't be any orbits; instead, all planetary bodies would simply float
around, running into each other when they crossed paths and just wandering forever.
There is a gravitational force between all objects in the universe. Gravitational force is what keeps all
components of our solar system in orbit around the Sun, as well as moons in orbit around planets. The
force of gravity affects Earth's tides and holds us on Earth's surface. The force of gravity between
objects depends on their masses and the distance between them.
Gravity is hard to understand and scientists have little to no understanding of how it works. We know
that gravity is out there, but the specifics are often lost on us. Plants, animals, and humans are all able
to grow tall due to the pull on Earth from the Sun. Without the Sun we would all just stretch out along
Earth's surface
None of the above
Answers
Answer:B
Explanation:
The summary which expresses the main points of the Gravitational force best is the second one.
What is gravitational force?Gravitational force is a attraction force which is present between two objects and represented as:
F = gm₁m₂ / r², where
g = gravitational constant
m₁ = mass of one object
m₂ = mass of another object
r = distance between two objects
Because of gravity all objects will have a accurate position and particular order.
Hence second paragraph expresses best.
To know more about gravitational force, visit the below link:
https://brainly.com/question/19050897
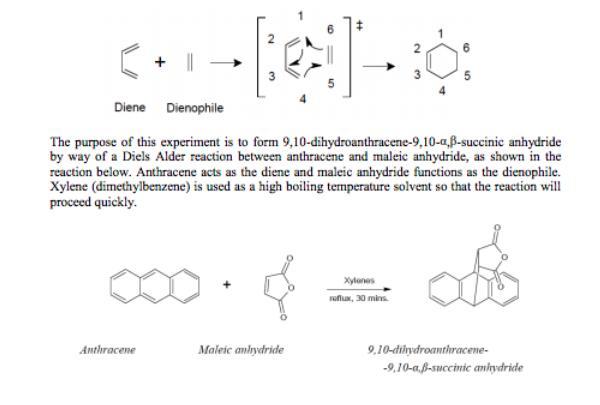
Consider the Diels-Alder reaction of anthracene and maleic anhydride. What is the role of each of the reactants
Answers
The role of each of the reactants are:
The o=benzene (extra line+C)=o acts as the [Dienophile]The three benzene rings acts as the [diene]What is the Diels-Alder reaction?
The Diels–Alder reaction is known to be a kind of reaction that is said to exist between what we call a conjugated diene and that of an alkene (dienophile).
Note that the union of both leads to a unsaturated six-membered rings and because the reaction is one that entails the formation of a cyclic product through what we call a cyclic transition state, it is said to be known as a "cycloaddition".
Note that from the experiment, The role of each of the reactants are:
The o=benzene (extra line+C)=o acts as the [Dienophile]The three benzene rings acts as the [diene]Learn more about Diels-Alder reaction from
https://brainly.com/question/14496475
#SPJ1
PLDD HELP
//////////////////////////////////////////////////////

Answers
Answer:chemical change
Explanation:
The volume of a gas is 204 ml when the pressure is 925 kPa at constant temperature what is the final pressure if the volume increases to 306 ml?
Answers
Calculate. 925 x 1.5 is 412.5 + 412.5 + 412.5.
This totals to 1387.5 kPa.
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
For every 1 mole of H2(g) produced, how many moles of electrons are transferred in the electrolysis of water
Answers
In an electrolytic cell, commonly made of platinum, electrolysis takes place between a positively charged anode and a negatively charged cathode.
Thus, Two half-reactions that take place at the cathode and anode make up the chemical reaction for water electrolysis. At the cathode, a reduction reaction takes place as hydrogen ions pick up electrons and change into hydrogen gas.
The separation of hydrogen and oxygen from water is demonstrated by the water electrolysis reaction.
Two moles of water are converted into two moles of hydrogen and one mole of oxygen. The amount of hydrogen produced is twice that of oxygen in moles. Between the electrodes and the electrolyte, charges are also exchanged.
Thus, In an electrolytic cell, commonly made of platinum, electrolysis takes place between a positively charged anode and a negatively charged cathode.
Learn more about Electrolyte, refer to the link:
https://brainly.com/question/29045708
#SPJ4
calculate the ph of the resulting solution if 34.0 ml34.0 ml of 0.340 m hcl(aq)0.340 m hcl(aq) is added to 24.0 ml24.0 ml of 0.440 m naoh(aq).
Answers
The pH of the resulting solution is approximately 1.81. I assumed that the volumes are additive and that the reaction between HCl and NaOH is complete.
The pH of a resulting solution can be calculated by considering the stoichiometry of the reaction between the acid (HCl) and the base (NaOH). The reaction between HCl and NaOH produces water and a salt, sodium chloride (NaCl).
To calculate the pH, we need to determine the concentration of the excess reactant after the reaction has occurred. In this case, HCl is in excess because its initial concentration is higher than that of NaOH.
Here are the steps to calculate the pH of the resulting solution:
1. Calculate the moles of HCl and NaOH used:
- Moles of HCl = volume (in L) × concentration (in mol/L) = 0.034 L × 0.340 mol/L = 0.01156 mol
- Moles of NaOH = volume (in L) × concentration (in mol/L) = 0.024 L × 0.440 mol/L = 0.01056 mol
2. Determine the limiting reactant:
- Since the moles of NaOH are less than the moles of HCl, NaOH is the limiting reactant. This means that all of the NaOH will react, and some of the HCl will be left unreacted.
3. Calculate the moles of HCl remaining:
- Moles of HCl remaining = moles of HCl initially - moles of NaOH used = 0.01156 mol - 0.01056 mol = 0.001 mol
4. Calculate the concentration of the HCl remaining:
- Concentration of HCl remaining = moles of HCl remaining / volume of solution (in L) = 0.001 mol / (0.034 L + 0.024 L) = 0.0154 mol/L
5. Calculate the pH using the concentration of HCl remaining:
- pH = -log[H+]
- [H+] = concentration of HCl remaining = 0.0154 mol/L
- pH = -log(0.0154) = 1.81 (rounded to two decimal places)
To know more about sodium chloride visit:-
https://brainly.com/question/14516846
#SPJ11
HELP ASAP WILL MARK BRAINLIEST

Answers
Answer:
Its C
Explanation:
At a temperature of 273K, a 400 mL gas sample has a pressure of 760 mm Hg. If the pressure is changed to 380 mmHg, at which temperature will this gas have a volume of 551mL?
Answers
Answer: The correct answer is 188.03K.
Explanation:
As a result, the gas sample will have a volume of 551mL at a temperature of 188.03K.
Section 1: Parts of Chemical Reaction and Conservation of mass
1) Identify the reactants cand products of the following
Chemical equation:
(The equation in image)

Answers
Answer:
The reactants are on the left of the arrow, the products are on the right.
Explanation:
Reactants are the substances that exist before the chemical reaction takes place. When writing a chemical reaction or equation, they are found on the left of the arrow. They react to form new substances, which are known as the products. The products are found to the right of the arrow in the reaction.
How does the valence electron configuration (entire last energy level configuration) relate to the group number on the Periodic Table? Use the shorthand notation in your discussion.
Answers
Answer:
The valence electron configuration determines the group to which an element belongs to.
Explanation:
The valence electron configuration of an atom of an element refers to the number of electrons in that atoms outermost shell.
The valence electron configuration is related to the group number of an element in that the number of electrons in the valence shell of an atom of an element determines the group to which an element belongs to. For example, all elements having one valence electron belong to group IA of the periodic table. Similarly, all elements having seven valence electrons belong to group VIIA of the periodic table.
This is because, since the reactivity of an element is related to its ability to either gain or lose a certain number of electrons in its valence shell, elements having same number of valence electrons have similar chemical properties.
What is co3 2- lewis structure?
Answers
Hence According to VSEPR theory, the CO32-Lewis structure has a trigonal planar molecular shape. The central carbon atom has also undergone sp2 hybridization.
As a result, the oxygen carbon oxygen (O-C-O) atoms have a 120 degree bond angle. In a trigonal planar structure with chemical symmetry D3h, it is made up of one carbon atom encircled by three oxygen atoms. The molecular weight of it is 60.01 g/mol Carbonate Ion (CO32) - Chemistry LibreTexts. Toggles Chapter Table menu, and a formal fee of two in total. Due to the sp2 hybridization of the carbon atom with the CO23 ion, the bond angle is 120°. Non-polar describes the carbonate ion.Resonance and its symmetry are responsible for this. Trigonal planar geometry is seen in the carbonate ion.
Learn more about hybridization here:
https://brainly.com/question/14140731
#SPJ4
Which of the following indicates if a reaction will proceed in reverse at any given conditions?
Group of answer choices
ΔGo > 0
ΔGo < 0
ΔG > 0
ΔG < 0
Answers
The main answer to your question is: ΔGo > 0 indicates if a reaction will proceed in reverse at any given conditions.
ΔGo (the change in Gibbs free energy) is a measure of spontaneity of a reaction.
If ΔGo is positive, it means that the reaction is not spontaneous and requires energy input to occur.
In this case, the reaction will tend to proceed in the reverse direction in order to minimize the free energy of the system.
Therefore, if ΔGo > 0, the reaction will proceed in reverse at any given conditions.
Summary: ΔGo > 0 indicates that a reaction will proceed in reverse at any given conditions.
Learn more about reaction click here:
https://brainly.com/question/11231920
#SPJ11
will give brainliest if you answer all of them

Answers
Answer:
Explanation:
A. Graphite can conduct electricity because of the delocalised (free) electrons in its structure. These arise because each carbon atom is only bonded to 3 other carbon atoms. ... However, in diamond, all 4 outer electrons on each carbon atom are used in covalent bonding, so there are no delocalised electrons.
B. Diamond is hard because the carbon atoms in diamond are bonded in a stronger tetrahedron pattern but graphite is soft and slippery because the carbon atoms in graphite are bonded in layers with only weak vanderwall force holding the layers together.
Phosphoric acid, h3po4, is a triprotic acid, for which ka1 = 7.2 × 10–3, ka2 = 6.3 × 10–8 and ka3 = 4.2 × 10–13. What is the value of kb for the hydrogen phosphate anion, hpo42–?
Answers
Answer:
Kb = 1.587x10⁻⁷
Explanation:
The equilibriums of phosphoric acid in water are:
H₃PO₄ ⇄ H₂PO₄⁻ + H⁺ Ka = 7.2x10⁻³
H₂PO₄⁻ ⇄ HPO₄²⁻ + H⁺ Ka = 6.3x10⁻⁸
HPO₄²⁻ ⇄ PO₄³⁻ + H⁺ Ka = 4.2x10⁻¹³
The Kb of HPO₄²⁻ is:
HPO₄²⁻ + H₂O ⇄ H₂PO₄⁻ + OH⁻
This equation is the result of the water equilibrium - Second Ka of phosphoric acid.
And Kb = Kw = Ka
Kb = 1x10⁻¹⁴ / 6.3x10⁻⁸
Kb = 1.587x10⁻⁷Given that H₃PO₄ is a triprotic acid for which Ka1 = 7.2 × 10⁻³, Ka2 = 6.3 × 10⁻⁸ and Ka3 = 4.2 × 10⁻¹³, the Kb for HPO₄²⁻ is 1.6 × 10⁻⁷.
Let's consider the 3 steps for the dissociation of phosphoric acid.
Step 1: H₃PO₄ + H₂O ⇒ H₂PO₄⁻ + H₃O⁺ Ka1
Step 2: H₂PO₄⁻ + H₂O ⇒ HPO₄²⁻ + H₃O⁺ Ka2
Step 3: HPO₄²⁻ + H₂O ⇒ PO₄³⁻ + H₃O⁺ Ka3
As we can see in the second step, HPO₄²⁻ is the conjugate base of H₂PO₄⁻. We can calculate its basic dissociation constant (Kb) using the following expression.
\(Kw = Ka2 \times Kb\\\\Kb = \frac{Kw}{Ka2} = \frac{1.0 \times 10^{-14} }{6.3 \times 10^{-8} } = 1.6 \times 10^{-7}\)
Given that H₃PO₄ is a triprotic acid for which Ka1 = 7.2 × 10⁻³, Ka2 = 6.3 × 10⁻⁸ and Ka3 = 4.2 × 10⁻¹³, the Kb for HPO₄²⁻ is 1.6 × 10⁻⁷.
Learn more: https://brainly.com/question/9728159
For the following reaction, K > 1. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases.
C9H7N + HNO2Doublearrow.GIFC9H7NH+ + NO2-
a) HNO2 1) stronger acid
b) NO2- 2) weaker acid
c) C9H7NH+ 3) stronger base
d) C9H7N 4) weaker base
Answers
For the given reaction with K > 1, we can classify the reactants and products based on their strength as Bronsted-Lowry acids or bases:
a) 1, b) 4, c) 2, d) 3.
C9H7N + HNO2 ⇄ C9H7NH+ + NO2-
a) HNO2 is a stronger acid (1) because it donates a proton to C9H7N.
b) NO2- is a weaker base (4) because it accepts a proton less readily compared to C9H7N.
c) C9H7NH+ is a weaker acid (2) because it donates a proton less readily compared to HNO2.
d) C9H7N is a stronger base (3) because it accepts a proton from HNO2.
To know more about Bronsted-lowry theory : https://brainly.com/question/15516010
#SPJ11
Help please help me

Answers
Answer: It completely dissociates in water is a characteristic of strong acid.
Explanation:
An acid which dissociates completely to give hydrogen ions \((H^{+})\) is called a strong acid.
For example, HCl is a strong acid and it dissociates completely as follows.
\(HCl \rightleftharpoons H^{+} + Cl^{-}\)
Strong acids are able to conduct electricity in water as more number of ions are present in the solution as compared to the ions present in a solution of weak acid.
Strong acids increase the concentration of \(H^{+}\) ions.
Thus, we can conclude that it completely dissociates in water is a characteristic of strong acid.
Help me please( ╹▽╹ )

Answers
Lower temperature
Let's verify
Pressure=Pvolume=VTemperature=TAs per Boyles law
\(\\ \rm\Rrightarrow V\propto \dfrac{1}{P}\)
As per Charles law
\(\\ \rm\Rrightarrow V\propto T\)
\(\\ \rm\Rrightarrow T\propto \dfrac{1}{P}\)
So
At higher altitudes lower the pressure so lower the temperature
for the structure, determine the total number of π electrons and the number of π electrons delocalized in the ring. indicate whether the compound is aromatic, nonaromatic, or antiaromatic. assume the structure is planar.
Answers
the total number of π electrons and the number of π electrons delocalized in the ring is 16 and 14. Compound is aromatic.
Assuming that the structure of the molecule is planar, we can use Hückel's rule to determine the number of pi electrons in the ring of the molecule. According to Hückel's rule, a planar, cyclic compound is aromatic if it has a continuous ring of p orbitals and the total number of pi electrons in the ring is equal to 4n + 2, where n is an integer.
For the molecule C14H12, if we assume that it is a planar, cyclic compound, we can draw its possible structures and count the number of pi electrons in the ring of each structure. Depending on the specific isomer, the number of pi electrons can vary.
The compound C14H12 corresponds to the molecular formula of several isomeric compounds, including several polycyclic aromatic hydrocarbons (PAHs) such as naphthalene, anthracene, and phenanthrene. These compounds contain a conjugated system of pi bonds, which can result in delocalization of pi electrons around the ring.
For more question on polycyclic aromatic hydrocarbons click on
https://brainly.com/question/15060847
#SPJ4
Question should be .click on image
