Answers
The pH of the 0.10 M NaOH solution is 13
How do i determine the ph of the solution?First, we shall determine the hydroxide ion concentration, [OH⁻] in the solution. Details below:
NaOH(aq) <=> Na⁺(aq) + OH⁻(aq)
From the above equation,
1 mole of NaOH contains 1 mole of OH⁻
Therefore,
0.10 M NaOH will also contain 0.10 M OH⁻
Next, we shall determine the pOH of the solution. details below:
Hydroxide ion concentration [OH⁻] = 0.10 MpOH of solution = ?pOH = -Log [OH⁻]
Inputting the various parameters, we have
pOH = -Log [OH⁻]
pOH = -Log 0.10
pOH = 1
Finally, we shall determine the pH of the solution. This is shown below:
pOH of solution = 1pH of solution = ?pH + pOH = 14
pH + 1 = 14
Collect like terms
pH = 14 - 1
pH = 13
Thus, the pH of the solution is 13
Learn more about pH:
https://brainly.com/question/22983829
#SPJ1
Related Questions
If the reaction occurs between Fe2S3(s) and H(aq) and the equation is properly balanced with the
smallest whole-number coefficients, what is the sum of the coefficients for all reactants and products?
Fe2S3(s) + H+ (aq)
Please answer this mathematically.
Answers
In the reaction between Fe₂S₃(s) and HBr(aq), the sum of the coefficients for all reactants and products is 12.
If the reaction occurs between Fe₂S₃(s) and HBr(aq) and the equation is properly balanced with the smallest whole-number coefficients, what is the sum of the coefficients for all reactants and products?
Let's consider the unbalanced equation for the reaction between Fe₂S₃(s) and HBr(aq). This is a double displacement reaction.
Fe₂S₃(s) + HBr(aq) ⇒ FeBr₃(aq) + H₂S(g)
We will balance it using the trial and error method.
First, we will balance Fe atoms by multiplying FeBr₃ by 2.
Fe₂S₃(s) + HBr(aq) ⇒ 2 FeBr₃(aq) + H₂S(g)
Then, we will balance S atoms by multiplying H₂S by 3.
Fe₂S₃(s) + HBr(aq) ⇒ 2 FeBr₃(aq) + 3 H₂S(g)
Finally, we will get the balanced equation by multiplying HBr by 6.
Fe₂S₃(s) + 6 HBr(aq) ⇒ 2 FeBr₃(aq) + 3 H₂S(g)
The sum of the coefficients for all reactants and products is:
\(1 + 6 + 2 +3 = 12\)
In the reaction between Fe₂S₃(s) and HBr(aq), the sum of the coefficients for all reactants and products is 12.
Learn more: https://brainly.com/question/7181548
A student needs to use the metric system while measuring the length of a box. Which units should he use?
AWNSER FAST PLEASEEE
Answers
Answer:
Centimeters or milimeters.
Explanation:
The answer depends on the size of the box.
The metric system base unit for length is the meter. Centimeters and milimeters are thus also part of the metric system.
If the box is a "regular-sized" box that we use to put stuff in (books, presents, etc), then centimeters would be the most appropiate.
If we're talking about those tiny boxes that are printed on paper (for example in some notebooks) then milimeters would be the most appropiate.
Answer:
Centimeters
Explanation:
50 points!
When a sodium thiosulfate solution (Na2S2O3(aq)) is added to hydrochloric acid, sulfur is formed.
1. What would you observe during the reaction? How could you set up the experiment to measure the reaction time for a given combination of thiosulfate and acid solutions?
2. What other products are formed?
3. Apart from heating it, how else could you speed up the reaction? How would this work?
Answers
Answer:
1. Insoluble yellow solid sulfur forms and gets deposited at the bottom. to setup the experiment draw a cross on a white paper and place beaker on top of it. take known volumes of sodium thiosulfate and hydrochloric acid in a beaker and mix it. At the same time start stop watch. measure time taken for the cross to disappear.
2. Sodium chloride, sulfurdioxide and water
3. increase concentration of acid
The isotope calcium-41 decays into potassium-41, with half-life of 1.03 x 105 years. There is a sample of calcium-41 containing 5 x 10° atoms. How
many atoms of calcium-41 and potassium-41 will there be after 4.12 › 105 years?
A.
3.125 x 108 atoms of calcium-41 and 4.375 x 10° atoms of potassium-41
6.25 x 108 atoms of calcium-41 and 4.6875 x 10° atoms of potassium-41
•
6.25 x 108 atoms of calcium-41 and 4.375 10° atoms of potassium-41
O D. 3.125 x 108 atoms of calcium-41 and 4.6875 › 10° atoms of potassium-41
Answers
3.125 × 10^8 atoms of calcium-41 and 4.6875 × 10^9 atoms of potassium-41.
Option D is correct
Isotope 41 of calcium is it?A rare and long-lived radioactive isotope of calcium is calcium-41 (41Ca).
# Given-
Half life = 1.03×10^5 years
- After 1.03×10^5 years (1 half life)
calcium-41 will be 50%
potassium-41 will be 50%
- After 2.06×10^5 years (2 half lives)
calcium-41 will be 25%
potassium-41 will be 75%
- After 3.09×10^5 years (3 half lives)
calcium-41 will be 12.5%
potassium-41 will be 87.5%
- After 4.12×10^5 years (4 half lives)
calcium-41 will be 6.25%
potassium-41 will be 93.75%
After 4.12×10^5 years,
Calcium-41 = 6.25/100 × 5 × 10^9 = 3.12×10^8 atoms
Potassium-41 = 93.75/100 × 5 × 10^9 = 4.69×10^9 atoms
To know more about Isotope 41 visit:-
https://brainly.com/question/29427598
#SPJ1
Identity the organic function group in the following?

Answers
We can see here that the organic function of cannabinol is its interaction with the endocannabinoid system (ECS) in the human body.
The organic function of procainamide lies in its ability to block sodium channels in cardiac cells, leading to a decrease in the excitability of the heart muscle and a suppression of abnormal electrical signals.
What is Procainamide?Procainamide is a medication used primarily in the treatment of cardiac arrhythmias, specifically ventricular arrhythmias. It belongs to a class of drugs known as antiarrhythmics, which work by regulating the electrical activity of the heart.
In addition to its antiarrhythmic properties, procainamide also possesses mild anticholinergic effects, which contribute to its overall pharmacological profile.
Cannabinol (CBN) is a cannabinoid compound found in the cannabis plant. While it is not as well-known as other cannabinoids like THC and CBD, it has some notable organic functions and potential effects on the body.
Learn more about Procainamide on https://brainly.com/question/31660681
#SPJ1
PROBLEM 19.12 Draw the structure of a triacylglycerol that fits each description: a. a saturated triacylglycerol formed from three 12-carbon fatty acids b. an unsaturated triacylglycerol that contains three cis double bonds c. a trans triacylglycerol that contains a trans double bond in each hydrocarbon chain
Answers
b. An unsaturated triacylglycerol that contains three cis double bonds would have three different unsaturated fatty acids attached to a glycerol backbone. Each fatty acid would contain a cis double bond.
c. A trans triacylglycerol that contains a trans double bond in each hydrocarbon chain would have three different trans fatty acids attached to a glycerol backbone. Each fatty acid would contain a trans double bond.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
Which of the following best describes a community?
• A. An interacting group of populations that occupy the same area
© B. A group of organisms that interbreed and have common
characteristics
• C. A group of organisms that interact with nonliving parts of the
environment
• D. All of the individuals of the same species that inhabit an
area
Answers
The following best describes a community
community is best describes as An interacting group of populations that occupy the same area
individuals of the equal species dwelling together contain a populationA network includes a doubtlessly interacting populations of different speciespopulation consists of individuals of the same species that live inside the same area, proportion the same habitat and sources, and probably interbreed with each other.A community and the nonliving elements with which it interacts is known as a ecosystemspecies is all of the populations of a particular type of organism.If a populace is experiencing exponential boom, it approach the larger the population, the faster it growsTo know more about population visit :
https://brainly.com/question/27991860
#SPJ9
What is heat transferred from movement of fluids in a circular motion called
Answers
Answer: it's known as heat transfer
Explanation: Heat always moves from a warmer substance to a cooler substance. For example, holding an ice cube will make your hand begin to feel cold in a few seconds. But is the coldness in the ice cube moving to your hand? No! Since cold is the absence of heat, it’s the heat in your hand that moves to the ice cube. This is one of the ways that heat is transferred.
According to the concept of thermal energy, heat transfer in a circular motion is called as convection.
What is thermal energy?
Thermal energy is defined as a type of energy which is contained within a system which is responsible for temperature rise.Heat is a type of thermal energy.It is concerned with the first law of thermodynamics.
Thermal energy arises from friction and drag.It includes the internal energy or enthalpy of a body of matter and radiation.It is related to internal energy and heat .It arises when a substance whose molecules or atoms are vibrating faster.
These vibrating molecules and atoms collide and as a result of which heat is generated in a substance , more the collision of particles , higher is the thermal energy.There are three types of thermal energy.
Learn more about thermal energy,here:
https://brainly.com/question/3022807
#SPJ6
Which structure is the Lewis structure for ammonia (NH3)?
A.
A bond line structure of a compound has N H H H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
B.
A bond line structure of a compound has H N H in the linear plane and hydrogen is branching upward, and the compound is H N (H) H.
C.
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
D.
A bond line structure of a compound has H N H H. The nitrogen has two dots on its top represents a lone pair of electrons.
Answers
Answer: **
H-N-H
|
H
Explanation:
Look at a periodic table to determine how many electrons you need to account for. Hydrogen (H) only has 1 electron, while Nitrogen (N) has 5. We have three Hydrogen atoms and one Nitrogen atom, so the total number of electrons will be 3 * 1 + 5 = 8 e-.
Now, place the center atom, which will be Nitrogen and place the three Hydrogens on three sides of it as above in the answer. You should use single bonds for this. Each single bond is a pair of electrons, so since we have three single bonds so far, we have accounted for 2 * 3 = 6 electrons. However, we need 2 more electrons for the total of 8. We put these electrons in as a lone pair above Nitrogen.
We check to see if everything follows the octet rule: Nitrogen has three single bonds, so that's 6 e-, as well as one lone pair, so that's another 2 e- for a total of 8 e-. Check. Now look at Hydrogen: H is the only element whose full orbital is 2 e-. Each H has a single bond with Nitrogen, so each does have 2 e-.
Thus, we know this is the correct diagram, and we are done.
Explanation:
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons. So ,the correct answer is option C.
The correct Lewis structure for ammonia (\(NH_3\)) is option C. It shows a bond line structure with three hydrogen atoms (H) bonded to a central nitrogen atom (N) in a linear plane.
One hydrogen atom branches upward from the plane. Additionally, the nitrogen atom in this structure has two dots at its bottom, indicating a lone pair of electrons. This arrangement follows the octet rule, as nitrogen has formed three covalent bonds with hydrogen, completing its valence shell. The lone pair on nitrogen gives ammonia its characteristic properties.
Thus, option C accurately represents the Lewis structure of ammonia, showing the bonding and lone pair arrangement of its atoms.
To know more about bond line structure:-
https://brainly.com/question/30639285
Which sense organ detects vibrations in the air. A. Ear B. Eye C. Tongue D. Nose
Answers
Match each tern with its definition by writing the letter of the correct definition on
the line beside tite term.

Answers
Answer:
3. d
4. c
5. i
6. h
7. a
8. g
9. j
10. b
11. e
12. f
Explanation:
how many grams of o2 are needed to produce 3.50 g of co2
Answers
Answer:
7 g of O2
Explanation:
For 1-C2 2-O2 are needed
3.50 + 3.50 = 7.00
please mark as brainlist answer
7 g is needed to produce 3.50 g of co2
The question can be solved as follows
= 3.50(2)
=7
Hence 7 g is needed to make 3.50 g of co2
Please see the link below for more information
https://brainly.com/question/22076117?referrer=searchResults
burning 12g of urea raise temp of water by 30C what is the enthalpy of combustion for 1kg urea
Answers
The enthalpy of combustion for 1kg of urea is -1223525.84 J/mol.
Urea is a compound that is used in fertilizers and in some plastics.The enthalpy of combustion for urea is the amount of energy that is released when urea is burned. In order to calculate the enthalpy of combustion for 1kg of urea, we need to use the information that is provided to us in the question. Let us start by writing down the balanced equation for the combustion of urea: CO(NH2)2 + 3/2 O2 → CO2 + 2H2O + N2
The balanced equation shows that 1 mole of urea reacts with 1.5 moles of oxygen gas to produce 1 mole of carbon dioxide, 2 moles of water, and 1 mole of nitrogen gas. The enthalpy change for this reaction is equal to the amount of energy that is released when 1 mole of urea is burned.
The heat of combustion (ΔHc) of urea is -632.6 kJ/mol. This means that 632.6 kJ of energy is released when 1 mole of urea is burned. We know that 12g of urea raised the temperature of water by 30°C. We can use this information to calculate the amount of energy that was released when 12g of urea was burned.
The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 J of energy to raise the temperature of 1 gram of water by 1°C. Therefore, it takes 4.18 x 1000 = 4180 J of energy to raise the temperature of 1 kg of water by 1°C.
We know that 12g of urea raised the temperature of water by 30°C. Therefore, the amount of energy that was released when 12g of urea was burned is:
Energy = mass x specific heat capacity x temperature change
Energy = 0.012 kg x 4180 J/kg°C x 30°C
Energy = 1497.6 J
We can now use this information to calculate the enthalpy of combustion for 1kg of urea:
Enthalpy of combustion = energy released / moles of urea burned
Enthalpy of combustion = 1497.6 J / (0.012 kg / 60.06 g/mol)
Enthalpy of combustion = - 1223525.84 J/mol
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
Which of the following is an incorrect representation for a neutral atom?
36Li
613C
3063Cu
1530P
Answers
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97. The incorrect representation for a neutral atom is 36Li
To determine the correct representation for a neutral atom, we need to consider the atomic number (Z) and mass number (A) of the element. The atomic number represents the number of protons in the nucleus, while the mass number represents the sum of protons and neutrons.
Let's analyze the given representations:
36Li:
This representation suggests that the element is lithium (Li) with a mass number of 36, which is incorrect. The correct mass number for lithium is approximately 6.94.
613C:
This representation suggests that the element is carbon (C) with a mass number of 13, which is correct. Carbon has different isotopes, and 13C represents one of its stable isotopes.
3063Cu:
This representation suggests that the element is copper (Cu) with a mass number of 63, which is correct. Copper has different isotopes, and 63Cu represents one of its stable isotopes.
1530P:
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97.
Therefore, the incorrect representation for a neutral atom is 36Li, as it does not match the known properties of lithium.
For more question on atom
https://brainly.com/question/26952570
#SPJ8
1.An object that produces electrical energy through redox reactions is called ____________.
oxidation
reduction
galvanic/voltaic cell
2.Which species undergoes oxidation?
Pb
Cu
Answers
\( \huge \bf༆ Answer ༄\)
Question : - 1
An object that produces electrical energy through redox reactions is called Galvanic cell
Question : - 2
Pb undergoes Oxidation
\(꧁ \: \large \frak{Eternal \: Being } \: ꧂\)
a student would like to use gravimetric analysis to determine the percent by mass of strontium chloride in a contaminated mixture containing magnesium chloride. which of the following compounds would be the best substance to react the contaminated mixture with to determine the mass percent?
Answers
Gravimetric analysis is a method of chemical analysis that relies on the measurement of mass in order to determine the composition of a sample. This technique is especially useful for determining the mass percent of a given compound in a sample, such as the strontium chloride in a contaminated mixture containing magnesium chloride.
In order to determine the mass percent of strontium chloride in a contaminated mixture, the best substance to react with the contaminated mixture is a substance that will selectively react with strontium chloride but not with magnesium chloride. An ideal reactant for this purpose is barium chloride, as it will form a precipitate with strontium chloride but not with magnesium chloride. The formation of the barium chloride precipitate can be used to determine the mass percent of strontium chloride in the contaminated mixture.
In order to perform the gravimetric analysis, the contaminated mixture must be reacted with barium chloride. The reaction should be carried out in a beaker containing the contaminated mixture and the required amount of barium chloride. The beaker should then be heated to boiling in order to facilitate the reaction. Once the reaction has been completed, the beaker should be allowed to cool and the precipitate should be filtered off, washed, and weighed. The mass of the precipitate can then be used to calculate the mass percent of strontium chloride in the contaminated mixture.
In conclusion, barium chloride is the best substance to react with a contaminated mixture containing both strontium chloride and magnesium chloride in order to determine the mass percent of strontium chloride. By reacting the contaminated mixture with barium chloride and measuring the mass of the resulting precipitate, the mass percent of strontium chloride in the mixture can be accurately determined.
Learn more about mass percent at :https://brainly.com/question/5394922
#SPJ4
which of the following nuclear equations has a correct characterization?
Answers
The correct answer is A.
The nuclear equation that correctly characterizes a nuclear reaction is one where the sum of the atomic numbers and the sum of the mass numbers on both sides of the equation are equal.
\(_{92}^{235}\textrm{U}+_{0}^{1}\textrm{n}\rightarrow _{54}^{140}\textrm{Xe}+_{38}^{94}\textrm{Sr}+3_{0}^{1}\textrm{n}\)
This conservation of both atomic and mass numbers ensures that the nuclear reaction obeys the laws of conservation of mass and conservation of charge.For example, consider the following nuclear equation:\(_{92}^{235}\textrm{U}+_{0}^{1}\textrm{n}\rightarrow _{54}^{140}\textrm{Xe}+_{38}^{94}\textrm{Sr}+3_{0}^{1}\textrm{n}\)
In this equation, the sum of the atomic numbers (92 + 0) and the sum of the mass numbers (235 + 1) on the left side are equal to the sum of the atomic numbers (54 + 38 + 0) and the sum of the mass numbers (140 + 94 + 3) on the right side. Therefore, this nuclear equation is correctly characterized and satisfies the conservation laws.The correct answer is A.
For such more question on nuclear equation
https://brainly.com/question/30743773
#SPJ8
Consider the nuclear equation below.
Which is the missing value that will balance the equation?

Answers
The balanced nuclear equation is as follows;
²²₁₁Na ---> ²²₁₀Ne + ⁰₋₁β
The correct answer is -1; option A.
What are nuclear equations?Nuclear equations are equations that show the changes that occur in the nucleus of atoms of elements.
The various types of nuclear reactions such as radiation decay, nuclear fusion, and nuclear fission are all represented by nuclear equations, which show the reactants and products. Atomic mass and proton number are conserved in nuclear reactions, as opposed to chemical equations, which demonstrate that the number of distinct elements is conserved in a reaction.
Learn more about nuclear equations at:
https://brainly.com/question/25387647
#SPJ1
An image of Earth's layer is above.
Which layer is the most dense?
layer A
layer B
layer C
layer D

Answers
Answer:
d
Explanation:
il answer in comments if you brainly me
How many atoms of phosphorus are in 8.80
mol of copper(II) phosphate?
Answers
By the concept of calculating moles ,it can br concluded that the no. of atoms of phosphorus in 8.80mol of copper(II) phosphate is=1.06\(x10^{25}\)
A mole is defined as amount of substance containing as many as elementary entities that are there in atoms of exactly 12 g of carbon-12.Therefore we can say,1 mole of copper(II) phosphate, Cu3(PO4)2, contains three moles of copper(II) cations and two moles of phosphate anions.
Again 1mole of phosphate anions contains one mole of phosphorus and four moles of oxygen.Considering all these informations we can conclude that:1 mole of copper(II) phosphate contains 2 moles of Phosphorus
Accordingle the sample contains=(8.80\(x\)2) moles of Phosphorus
=(17.6\(x\)Avogadro's constant) atoms of Phosphorus
=(17.6\(x\)6.022\(x\)\(10^{23}\)) atoms of Phosphorus
=1.06\(x10^{25}\) [approx] no. of Phosphorus atoms
to learn more about Moles,
https://brainly.com/question/30892840
In 8.80 moles of copper(II) phosphate, there are approximately 1.06 x 10²⁵ atoms of phosphorus.
Explanation:The number of atoms of phosphorus in a given amount of a compound can be calculated using the concept of mole in chemistry. Copper(II) phosphate is Cu3(PO4)2, containing 2 moles of phosphorus (P) for every 1 mole of the compound. Avogadro's number (6.022 x 10²³) gives the number of atoms in one mole.
So, if there are 8.80 moles of copper(II) phosphate, there would be 2 × 8.80 moles of phosphorus. Multiplying this by Avogadro's number gives the total number of phosphorus atoms.
Therefore, the number of phosphorus atoms is 2 × 8.80 × 6.022 x 10²³ = 1.06 x 10²⁵ atoms of phosphorus.
Learn more about Moles to atoms conversion here:https://brainly.com/question/32787463
#SPJ2
What do you think is the strongest chemical bonding?why? OWN PERSPECTIVE PLS!!
Answers
explanation: answer on safari hope this helps!
How many valence electrons are in the electron dot structures for the elements in group 3A(13)?
Answers
Answer:
here, as we have known the elements of group 3A(13) such as aluminium , boron has three valance electron and in perodic table the elements are kept with similar proterties in same place so, their valance electron is 3.
hope it helps...
The number of valence electrons are in the electron dot structures for the elements in group 3A(13) is three.
What are Groups in the Periodic Table?The periodic table is organized into groups (vertical columns), periods (horizontal rows), and families (groups of elements that are similar). Elements in the same group have the same number of valence electrons.
Groups are the columns of the periodic table, and periods are the rows. There are 18 groups, and there are 7 periods plus the lanthanides and actinides.
There are two different numbering systems that are commonly used to designate groups, and you should be familiar with both.
The traditional system used in the United States involves the use of the letters A and B. The first two groups are 1A and 2A, while the last six groups are 3A through 8A. The middle groups use B in their titles.
Therefore, The number of valence electrons are in the electron dot structures for the elements in group 3A(13) is three.
Learn more about Groups in the periodic table, here:
https://brainly.com/question/30858972
#SPJ3
I need help calculating the error % in molar mass
Answers
Error % = |(experimental - actual) / actual| x 100%
For example, let's say the actual molar mass of a compound is 100 g/mol, and the experimental molar mass determined in the lab is 95 g/mol. The error percentage would be:
Error % = |(95 - 100) / 100| x 100%
Error % = |-0.05| x 100%
Error % = 5%
Therefore, the error percentage in molar mass is 5%
Which of the following substances would you expect to have a low melting point?
A. carbon dioxide
B. silicon dioxide
C. lithium chloride
D. calcium bromide

Answers
Ionic compounds have higher melting point than covalent compounds.
Carbon dioxide have lower melting point (-56.6 °C) among the given compounds.
What is melting point?Melting point is the temperature at which the substance melts into liquid thus at this temperature its solid state and liquid state are in equilibrium. Melting point is depend upon the bond type and mass of the compound.
Ionic bonds are stronger than covalent bonds and hence ionic compounds have higher melting point than covalent compounds. Hence, lithium chloride and calcium bromide have higher melting point.
Silicon is bigger than carbon thus silicon dioxide melts at higher temperature. Carbon dioxide is purely covalent and melts at negative temperatures.
Hence, the compound with lower melting point here is carbon dioxide thus, option A is correct.
To learn more about melting point. refer the link below:
https://brainly.com/question/5753603
#SPJ2
PLZ HELP PLZ HURRY NO WRONG ANSWER PLZ ALSO WILL GIVE BRAINLIEST
An atom of an element contains 6 protons, 6 neutrons, and 6 electrons. An atom that is a different isotope of this element would contain —
A.
6 protons, 7 neutrons, and 6 electrons.
B.
6 protons, 6 neutrons, and 7 electrons.
C.
7 protons, 6 neutrons, and 6 electrons.
D.
7 protons, 7 neutrons, and 7 electrons.
Answers
Answer:
I dont lnow much about chemistry, but I believe it's either d or a. sorry if its wrong!
Eaterfication experiment
Answers
Answer:
Do you mean Esterification experiment?
Explanation:
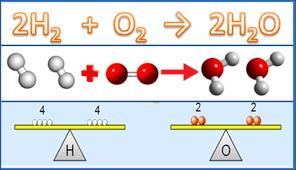
Hydrogen gas (h2) and oxygen gas (o2) combine. Which change occurs that indicates a release of bond energy?.
Answers
The creation of water (H2O), as well as the production of heat and light, signal the release of bond energy when hydrogen gas (H2) combines with oxygen gas (O2).
Bond Energy, commonly referred to as average bond enthalpy or just bond enthalpy, is a measurement that provides information about how strong a chemical bond is. "The average value determined from the bond dissociation enthalpies (in the gaseous phase) of all the chemical bonds of a certain type in a given chemical compound," is how the word "bond energy" is defined by the IUPAC. As a result, the average amount of energy needed to break one of these chemical bonds may be thought of as the bond energy of a chemical bond in a specific molecule.
Learn more about Bond Energy.
brainly.com/question/17514510
#SPJ4
Choose the best answer for this question.
Describe way(s) that your speed could change as you jog along a park's path.
O speed up
all of these, except none of these
O none of these
O slow down
Answers
Your speed could change in several ways as you jog along a park's path. Option 2.
Speed of joggingOne possible way is that you could speed up if you increase your pace or start running instead of jogging. This could happen if you feel more energized, motivated, or if you need to catch up with someone.
Conversely, your speed could slow down if you get tired, experience muscle fatigue, or encounter an uphill section of the path that requires more effort. Other factors such as weather conditions, terrain, or distractions can also affect your speed.
Therefore, the correct answer would be "all of these, except none of these," as there are various factors that can cause changes in your jogging speed along a park's path.
More on speed can be found here: https://brainly.com/question/14969657
#SPJ1
