draw the structure of the major product formed in the given reaction when the aromatic aldehyde is present in excess. an aldehyde and ester react with methoxide in methanol. the aldehyde consists of two fused benzene rings. if the top fused carbon is arbitrarily numbered 1 and the ring numbered clockwise, there is an aldehyde substituent on carbon 3 and a methoxy substituent on carbon 8. the ester is a carbonyl bonded to methyl and methoxy.
Answers
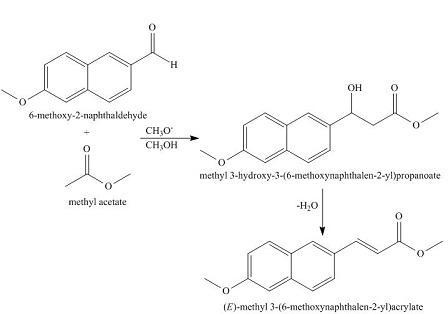
When combined with methyl acetate, the chemical 6-methoxy-2 naphthaldehyde undergoes an aldol addition reaction to produce methyl 3-hydroxy-3-(6-methoxynaphthalen-2-yl)propanoate. The aldol addition product undergoes dehydration to create the aldol condensed product, (E)-methyl 3-(6-methoxynaphthalen-2-yl)acrylate.
What is aldol condensation reaction?
In an aldol condensation, an enolate ion combines with a carbonyl molecule in the presence of an acid/base catalyst to produce either a hydroxylated aldehyde or ketone, which is then dehydrated to produce a conjugated enone. It is an effective process for creating carbon-carbon bonds.
To learn more about aldol condensation reaction click the given link
https://brainly.com/question/27178362
#SPJ4
Related Questions
Explain how a mutation becomes an adaptation.
Answers
Answer:
An adaptation occurs if a mutation helps an individual survive and reproduce.
Explanation:
Over generations, the mutation will become more common. An adaptation occurs if a mutation isn't passed on to offspring. Hope this helps! xx
To calculate the charge of an atom you must
Answers
Answer:
The charge of an element is equal to the number of protons minus the number of electrons. The number of protons is equal to the atomic number of the element given in the periodic table. The number of electrons is equal to the atomic number minus the charge of the atom.
Explanation:
What is the name of this molecule?
H3C - C ≡ C - CH3
Answer: 1,2,3, or 4-butyne?
Answers
Explanation:
The given molecule is
H3C - C ≡ C - CH3
The numbering order is shown below:
H3C - C ≡ C - CH3
1 2 3 4
So, the alkyne group is in the second position.
The carbon chain has four carbons.
Hence, the IUPAC name of the given compound is:
2-butyne.
ph of a solution of a strong acid is 5.0. what will be the ph of the solution obtained after diluting the given solution a 100 times?
Answers
The pH of the solution obtained after diluting the original solution by 100 times will be 7.
This is because the hydrogen ion concentration will decrease from 10⁻⁵ M to 10⁻⁷ M, resulting in an increase of the pH value.
As the concentration of acid molecules decreases, the pH of the solution increases. This is because the pH scale is logarithmic, so a small change in hydrogen ion concentration results in a large change in pH. Additionally, the pH of a solution can be affected by other factors such as the presence of buffers, which act to keep the pH of the solution stable.
Learn more about ph of a solution:
https://brainly.com/question/26424076
#SPJ4
What volume of "laughing gas" (N2O) will be produced from 60 g of nitrogen gas (N2) and 85 g of oxygen gas (O2)? How much of the excess reactant will be left? 2N2 + O2 -> 2N2O
Answers
Answer:
94.16g of N2O
50.88g of O2
Explanation:
O2 reacts with N2 as follows:
2N2 + O2 → 2N2O
To find moles of N2O produced we need to convert the mass of each gas to moles to find limiting reactant and the moles of N2O that could be produced:
Moles N2 (Molar mass: 28g/mol):
60g N2 * (1mol / 28g) = 2.14 moles N2
Moles O2 (Molar mass: 32g/mol):
85g O2 * (1mol / 32g) = 2.66 moles O2
For a complete reaction of 2.14 moles of N2 are needed:
2.14 mol N2 * (1mol O2 / 2 mol N2) = 1.07 moles of O2.
As there are 2.66 moles of O2, reactant in excess is O2 and will remain:
2.66 moles - 1.07 moles =
1.59 moles O2. The mass is:
1.59 moles O2 * (32g / mol) =
50.88g of O2And there are produced:
2.14 mol N2 * (2 moles N2O / 2 moles N2) = 2.14 moles N2O.
The mass is (44g/mol for N2O):
2.14 moles N2O * (44g/mol) =
94.16g of N2Owhich analysis technique could be used to differentiate among these three glycans and determine the structure of each if they were present in a mixed sample? choose one:
Answers
One analysis technique that could be used to differentiate among these three glycans and determine the structure of each if they were present in a mixed sample is mass spectrometry (MS).
One analysis technique that can be used to differentiate among the three glycans and determine the structure of each if they were present in a mixed sample is mass spectrometry.
Mass spectrometry is a powerful analytical technique that can provide information on the molecular mass, structure, and composition of a molecule. In the case of glycans, mass spectrometry can be used to identify the number and type of monosaccharides, the presence and location of modifications (such as acetylation or sulfation), and the branching pattern of the glycan.
By analyzing the mass spectra of the individual glycans in the mixed sample, it is possible to differentiate among the three glycans and determine the structure of each.
To know more about mass spectrometry refer here:
https://brainly.com/question/26500669
#SPJ11
In Part I, why do we use different concentrations for sulfuric acid and sodium hydroxide, 3 M H2SO4 versus 6 M NaOH? Grading guidelines: -0.5 pts - A reference to the significance of concentration and possible difference between the acid and base are given. -O pts - No reference to the concentrations
Answers
In Part I, we use different concentrations for sulfuric acid (3 M H₂SO₄) and sodium hydroxide (6 M NaOH) due to their differences in chemical properties and reactivity.
The main reason for using different concentrations is to achieve the desired neutralization reaction between the acid and the base. Sulfuric acid is a strong diprotic acid, meaning it can donate two protons (H+ ions) per molecule. On the other hand, sodium hydroxide is a strong monoprotic base, meaning it can accept only one proton per molecule.
Using a higher concentration of NaOH (6 M) ensures that there are enough hydroxide ions (OH⁻) to react with the two protons donated by each H₂SO₄ molecule at the 3 M concentration. This allows the neutralization reaction to proceed effectively, forming water and a salt as products.
The different concentrations of 3 M H₂SO₄ and 6 M NaOH are chosen to account for the differences in chemical properties and reactivity between the acid and base, ensuring an efficient neutralization reaction.
To know more about sulfuric acid, refer
https://brainly.com/question/10220770
#SPJ11
what is the ph of a 0.753 m (ch3)3nhcl aqueous solution at 25°c? kb for (ch3)3n = 6.4 x 10−5.
Answers
The final answer is pH = 8.47. The pH of a 0.753 M (CH3)3NHCl aqueous solution at 25°C can be calculated using the equation pH = pKa + log([base]/[acid]), where pKa is the negative logarithm of the acid dissociation constant, [base] is the concentration of the base, and [acid] is the concentration of the acid.
In this case, (CH3)3NHCl is a salt that will hydrolyze in water to produce (CH3)3NH, which acts as a base, and HCl, which acts as an acid.
The Kb for (CH3)3N is given, so we can find the pKa using the equation pKa + pKb = 14. From there, we can use the concentrations of (CH3)3NH and HCl to calculate the pH of the solution.
To know more about negative logarithm refer here:
https://brainly.com/question/3072484#
#SPJ11
Calculate the change of enthalpy for the reaction 2Al (s) + 3Cl2 (g) --> 2AlCl3 (s) from the following reactions
Reaction 1: 2Al (s) + 6HCl (aq) --> 2AlCl3 (aq) + 3H2 (g) Change in enthalpy: -1049 kJ
Reaction 2: HCl (g) --> HCl (aq) Change in enthalpy: -74.8 kJ/mol
Reaction 3: H2 (g) + Cl2 (g) --> 2HCl (g) Change in enthalpy: -1845. kJ/mol
Reaction 4: AlCl3 (s) --> AlCl3 (aq) Change in enthalpy: -323. kJ/mol
Include the following:
The numerical answer with correct units.
State which reactions, if any, you had to "Flip".
State which reactions you had to multiply, if any, to get the correct amount of the compound. Also, include how much you multiplied the reaction by.
Answers
The change of enthalpy for the given reaction is equal to -6387 KJ.
What is the enthalpy change?Enthalpy can be defined as the sum of internal energy and the product of volume and Pressure, expressed as shown below:
H = U + PV
The change in enthalpy between the beginning and final states can be expressed as:
ΔH = ΔU + ΔPV
Given reactions with enthalpy change are shown below:
2Al (s) + 6HCl (aq) → 2AlCl₃ (aq) + 3H₂ (g) }×1 ΔH₁ = -1049 kJ
HCl (g) → HCl (aq) } ×6 ΔH₂ = -74.8 kJ/mol
H₂ (g) + Cl₂ (g) → 2HCl (g) }× 3 ΔH₃ = -1845. kJ/mol
AlCl₃ (aq) → AlCl₃ (s) }× 2 ΔH₄ = +323. kJ/mol
\(\triangle H^o_f =1\times \triangle H_1 +6\times \triangle H_2+3\times \triangle H_3+2\times \triangle H_4\)
\(\triangle H^o_f =1\times (-1049) +6\times (-74.8)+3\times (-1845)+2\times (+323)\)
\(\triangle H^o_f =-6387 KJ\)
Therefore, the change of enthalpy for the reaction of the formation of aluminum chloride is -6387 KJ.
Learn more about enthalpy change, here:
brainly.com/question/4526346
#SPJ1
□
5. Which of the following measurements have four significant figures? Select all that apply.
5,000 s
120.0 mL
0.0007 cm
1,001 g
Answers
Answer:
1001
Explanation:
Zeroes between non zero digits are significant
Answer:
1001 g and 120.0 mL
Explanation:
Zeros in-between natural numbers are significant
If your number has a decimal place, and you have zeroes after a natural number, then all the zeros are sig figs.
Ex: 10000.0 = 6 sig fig
Not: 0.00001 = 1 sig fig
Not: 0012.0 = 3 sig fig
The atomic number identifies which of the following?
a
number of neutrons
b
number of electrons and neutrons
c
number of protons
d
number of protons and neutrons
Answers
Answer: protons
Explanation:
The atomic # identifies the amount of protons in an atom
What subatomic particles cause the mass of the atom to change?
Answers
Subatomic particles that cause the mass of an atom to change are called isotopes. Isotopes are atoms of the same element that have an unequal number of neutrons in their nuclei.
This difference in the number of neutrons causes the mass of the atom to vary. For example, the most common form of carbon is Carbon-12, which has an atomic mass of 12 and contains 6 protons and 6 neutrons, while Carbon-14 has an atomic mass of 14 and contains 6 protons and 8 neutrons. The extra two neutrons make Carbon-14 slightly heavier than Carbon-12. The number of neutrons in an atom is determined by the number of protons and the element's atomic number, so when the number of neutrons changes, the mass of the atom changes as well. Isotopes can be naturally occurring or artificially created, and their properties may vary depending on the number of neutrons in the nucleus. Overall, isotopes are subatomic particles that cause the mass of the atom to change.
To learn more about isotopes click here https://brainly.com/question/21536220
#SPJ4
HELP ASAP!!
Two colorless chemicals combine inside a glow
stick. When the chemicals combine, they produce
brightly colored light. The glow stick's temperature
does not change. (select all that apply)
emission of heat
emission of light
color change
formation of ga
Answers
Answer:
Emission of light
color change:)
Explanation:
Answer:
B and C
Explanation:
edge 2021
I just did it
when removing successive outermost electrons from sodium. the first ionization energy is 495 kj/mol, while the second ionization energy is 4562 kj/mol. however, when removing successive electrons from magnesium, the first ionization energy is 738 kj/mol and the second ionization energy is 1451 kj/mol. why is there a bigger increase in second ionization energy for sodium than there is for magnesium?
Answers
There a bigger increase in second ionization energy for sodium than there is for magnesium because fully filled orbitals have extra stability.
The electronic configuration of Sodium is:
Na = 1s2 2s2 2p6 3s1
After removing one electron, the electronic configuration is
Na⁺ = 1s2 2s2 2p6 ( I.E 1 = 495)
I.E 1 of sodium = 495 and I.E 2 of sodium = 4562
I.E 1 < I.E 2 because half filled and fully filled orbitals have extra stability.Also , I.E 2 is very high because an electron is removed from fully filled p orbital. After losing an electron , sodium attains stable noble gas configurationThe electronic configuration of Magnesium is:
Mg = 1s2 2s2 2p6 3s2
There a bigger increase in second ionization energy for sodium than there is for magnesium because:
The electronic configuration of Na⁺ is Ne and electronic configuration of Mg⁺¹ is [Ne] 3s1 so removing an electron from 3s takes less energy than removing an electron from 2pSince Na⁺ ion is larger than Mg⁺ ion , it takes more energy to remove an electron from sodium ionLearn more about ionization energy at https://brainly.com/question/1445179
#SPJ4
how does the law of conservation of mass also apply to physical changes?
Answers
The law of conservation of mass states that the total mass of a system remains constant, regardless of the changes that occur within the system. This means that the total mass of the products of a physical change must be equal to the total mass of the reactants.
For example, consider the process of melting a block of ice. When the ice melts, it changes from a solid to a liquid, but its total mass remains the same. The mass of the water that results from the melting of the ice must be equal to the mass of the ice that was present before the change.
Similarly, other physical changes such as evaporation, condensation, sublimation, and freezing also follow the law of conservation of mass. In each of these processes, the total mass of the system remains constant, even though the physical state of the substance changes.
In conclusion, the law of conservation of mass applies to physical changes as well as chemical reactions, and requires that the total mass of the system remains constant, regardless of the changes that occur within the system.
To learn more about law of conservation of mass visit here:
https://brainly.com/question/28711001#
#SPJ11
ased on what you did and learned in today's lab what can you predict if 1- propanol is mixed with distilled water? Are the mass and volume conserved?
Answers
Based on what we did and learned in today's lab, we can predict that when 1-propanol is mixed with distilled water, the mass and volume will be conserved.
This is because 1-propanol and water are both liquids and when they are mixed together, they will form a homogeneous solution. A homogeneous solution is a mixture of two or more substances that have a uniform composition and appearance throughout the entire mixture.
The mass of the solution will be the sum of the masses of the two liquids, and the volume of the solution will be the sum of the volumes of the two liquids. Therefore, the mass and volume will be conserved in this case.
To learn more about Volume :
https://brainly.com/question/29796637
#SPJ11
Which of the following is NOT a part of adenosine diphosphate?
glucose, ribose, adenine, two phosphate groups
Answers
Answer:
a. glucose c. ribose b. adenine d. two phosphate groups user: all organisms need energy to perform different functions. cells are able to ...
4.
How many grams would .50 moles of Mg be?
5.
10 moles of Al = ? atoms
6.
How many atoms are in 10 moles of Zinc?
7.
If you had 3,5 x 10 - atoms of carbon how many grams would you have?
8.
Given 100g of C = ? atoms
Answers
Answerl
see explanation
Explanation:
Question
4.
How many grams would 0 .50 moles of Mg be?
Mg has an atomic weight of 24 so 1 mole of Mg weighs 24 gms
0.50 moles weigh 0.50 X24gm = 12 gm
5.
10 moles of Al = ? atoms
1 mole = 6.02 X10^23 atoms ...Avogadros number
10 moles is 10 X6.02X10^23 = 6.02X10^24
6.
How many atoms are in 10 moles of Zinc?
a mole is Avogadros number
same as 10 moles of Al,10 moles of Zn= 6.02 X10^24 atoms
7.
If you had 3,5 x 10 - atoms of carbon how many grams would you have?
what is the power of the 10-in the question...it was cut off and the question cannot be answered as isif it was 3.5 x10^23 that would be 3.5/6.02 =0,581 moles of C and would weigh0.581X12 = 6.97 gm,
8.
Given 100g of C = ? atoms
Carbon has an atomic of 12 100 gm of C are 100/12 = 8.33 moles of C
each mole comtains Avogadros number so there are
8.33 X 6.02 X10^23 = 5.01 X10^24 atoms of C
What is the difference between criminology and forensic science?
Criminology analyzes how crime affects the community or society as a whole, while forensic science analyzes individual crimes and evidence
O Criminology is an applied Science, while forensic science is an academic science
O Criminologists work closely with law enforcement, while forensic scientists work with academic institutions
o Criminology is a type of forensic science
Answers
Answer:
pick the second one I've done that test
Answer:
Criminology analyzes how crime affects the community or society as a whole , while forensic science analyzes individual crimes and evidence.
Determine the electronegativity difference (AEN) and the type of bond formed
(ionic, polar, non-polar).
CF4

Answers
the electronegativity difference (AEN):
<0.5: covalent (non polar)
0.5- 1.7: covalent (polar)
> 1.7 : ionic
CF₄
C = 2.5
F = 4
F - C = 4 - 2.5 = 1.5 (covalent polar)
NH₃
N = 3
H = 2.1
N - H = 3 - 2.1 = 0.9 (covalent polar)
ohesion between water molecules contributes to __________ which allows the molecules to stick together and resist outside forces.
Answers
Answer: I believe the answer is surface tension :)
What is the pH of the final solution when 50.0 g NH4Cl(s) is dissolved in 1.0 L of a 0.50 M NH3 solution
Answers
NH₄Cl and NH₄CN will make the solutions slightly acidic, while H₂CO₃, KOH, and KClO₄ will result in basic or neutral solutions.
1. The pH of the final solution will be slightly basic, around 9. This is because NH₄Cl is a salt of a weak acid (NH₃) and a strong base (HCl). When NH₄Cl dissolves in water, it will dissociate into NH₄⁺ and Cl⁻ ions. The NH₄⁺ ions will react with the OH⁻ ions from the water to form NH3 and H₂O. This will increase the concentration of OH⁻ ions in the solution, making it basic.
2. The pH of the final solution will be 10. This is because the H₂CO₃ is a weak acid and the KOH is a strong base. When the two solutions are mixed, the H₂CO₃ will react with the KOH to form K₂CO₃ and H₂O. The K₂CO₃ is a salt that does not hydrolyze, so it will not affect the pH of the solution. The only ions in solution will be K⁺ and OH⁻, which will make the solution basic.
3. The pH of the final solution will be 13. This is because KOH is a strong base. When KOH dissolves in water, it will dissociate completely into K⁺ and OH⁻ ions. The OH⁻ ions will make the solution basic.
4. The pH of a 0.50 M H₃PO₄ solution will be 2.1. This is because H₃PO₄ is a strong acid. When H₃PO₄ dissolves in water, it will dissociate completely into H⁺ and H₂PO₄⁻ ions. The H⁺ ions will make the solution acidic.
5. The pH of a solution of NH₄CN will be < 7. This is because NH₄CN is a salt of a weak acid (NH₄⁺) and a weak base (CN⁻). When NH₄CN dissolves in water, it will dissociate into NH₄⁺ and CN⁻ ions. The NH₄⁺ ions will react with the OH⁻ ions from the water to form NH₃ and H₂O. This will decrease the concentration of OH⁻ ions in the solution, making it acidic.
6. The pH of a solution of KClO₄ will be 7. This is because KClO₄ is a salt of a strong acid (HClO₄) and a strong base (KOH). When KClO₄ dissolves in water, it will dissociate completely into K and ClO₄⁺ ions. The K⁺ and ClO₄⁻ ions will not react with the water, so they will not affect the pH of the solution. The pH of the solution will be determined by the concentration of H⁺ ions in the water, which is 10⁻⁷ M.
To know more about the pH of the final solution refer here,
https://brainly.com/question/11867446#
#SPJ11
Complete question :
What is the pH of the final solution when 50.0 gNH_4 Cl_(s) dissolved in 1.0 L of a 0.50 M NH_3 solution? (Assume NO CHANGE IN VOLUME) What is the pH of the final solution when 50.0 mL of 0.10 M H_2 CO_3 solution is mixed with 25.0 mL of a 0.20 M KOH solution? What is the pH of a solution made by dissolving 112.2 g KOH_(s) in enough water to make 1.0 L. What is the pH of a 0.50 M H_3 PO_4 solution? Will the pH of a solution of NH_4 CN be > 7, < 7, or = 7? MUST show reasoning. Will the pH of a solution of KClO_4 be > 7, < 7, or = 7? MUST show reasoning.
how many liters of a 0.20 % (m/v) kcl iv solution can be prepared from 3.0 l of a 5.0 % (m/v) stock solution?
Answers
75 liters of a 0.20% (m/v) KCl IV solution can be prepared from 3.0 L of a 5.0% (m/v) stock solution.
To determine the amount of a 0.20% (m/v) KCl IV solution that can be prepared from a 5.0% (m/v) stock solution, the following formula can be used:
C1V1 = C2V2
where C1 is the concentration of the stock solution, V1 is the volume of the stock solution used, C2 is the desired concentration of the final solution, and V2 is the volume of the final solution.
In this case, C1 = 5.0%, V1 = 3.0 L, C2 = 0.20%, and V2 is what we are trying to find.
First, convert the percentages to decimals:
C1 = 0.050
C2 = 0.0020
Now we can plug in the values and solve for V2:
(0.050)(3.0) = (0.0020)(V2)
0.15 = 0.0020V2
V2 = 75 L
Therefore, 75 liters of a 0.20% (m/v) KCl IV solution can be prepared from 3.0 L of a 5.0% (m/v) stock solution.
Learn more about stock solution.
https://brainly.com/question/25256765
#SPJ4
75 liters of a 0.20% (m/v) KCl IV solution can be prepared from 3.0 L of a 5.0% (m/v) stock solution.
To determine the amount of a 0.20% (m/v) KCl IV solution that can be prepared from a 5.0% (m/v) stock solution, the following formula can be used:
C1V1 = C2V2
where C1 is the concentration of the stock solution, V1 is the volume of the stock solution used, C2 is the desired concentration of the final solution, and V2 is the volume of the final solution.
In this case, C1 = 5.0%, V1 = 3.0 L, C2 = 0.20%, and V2 is what we are trying to find.
First, convert the percentages to decimals:
C1 = 0.050
C2 = 0.0020
Now we can plug in the values and solve for V2:
(0.050)(3.0) = (0.0020)(V2)
0.15 = 0.0020V2
V2 = 75 L
Therefore, 75 liters of a 0.20% (m/v) KCl IV solution can be prepared from 3.0 L of a 5.0% (m/v) stock solution.
Learn more about stock solution.
brainly.com/question/25256765
#SPJ11
What problems might it cause if a company tried to recycle materialswithout sorting them first?
Answers
What is the purpose of adding sodium sulfate to the organic layer after extraction?.
Answers
An electric drill pulls 1000 joules per second or electrical energy from
the wall. The drill bit only exerts 555 joules of energy on the wood it's
drilling through. Where did all the extra energy go?
Answers
All of the energy stored or applied on a body cannot be converted tono work done only the available form of energy is converted to work. The remaining part is called unavailable energy which is stored on the body or taken as increase in entropy.
What is unavailable energy?According to thermodynamic concepts, the not all the energy can be converted into useful work .Or in other words only available energy can be converted to work. This available form of energy is called Gibbs energy.
For instance if we take a heat engine, the heat from the hot body is not completely transferred into colder body. According to third law of thermodynamics, this unavailable work is being considered as gone for increase in entropy.
Therefore, only available energy of the electric drill can be changed to work done and that is 222 J out of 1000 J, remaining goes for an increase in entropy of the system.
To find more on entropy, refer here:
https://brainly.com/question/24278877
#SPJ1
If I have 4.1 moles of nitrous oxide (laughing gas) that is kept at 24.5°C in a container under 2.2 atm, then what is the volume of the container? [R = 0.0821 (L*atm)/(mol*K)]
Answers
We have a gas that we will assume behaves like an ideal gas, so we can apply the ideal gas equation.
\(PV=nRT\)Where,
V is the volume of the gas
P is the pressure of the gas=2.2atm
T is the temperature of the gas = 24.5 + 273.15K=297.65K
R is a constan=0.0821atm.L/mol.K
n is the number of moles = 4.1moles
Now, we clear V and replace the known data:
\(V=\frac{nRT}{P}\)\(\begin{gathered} V=\frac{4.1mol\times0.0821\frac{atm.L}{mol.K}\times297.65K}{2.2atm} \\ V=45.5L \end{gathered}\)The volume we find is the volume that the gas occupies. Now, gases due to their characteristics occupy the volume of the container where they are contained. Therefore, the volume of the container will also be 45.5L.
Answer: The volume of the container is 45.5L
The concentration of lactic acid in the
yogurt sample is 0.01 M.
What mass of lactic acid is present in the
40.00 mL sample?
Answers
Answer:
C=m/MV
Explanation:
m= CMV= .01×40×10³=
4000g
Shown in the table below are solubility data for different salts in water at 60oC.
Which salt is most soluble at 60o C?
A, B, C, or D.
(Chart Below:)

Answers
Answer:
D is the most soluble in water at 60 degrees Celsius
According to the data given in the table, the salt is most soluble at 60o C - salt D.
In the given table it is given that all the salts are soluble in different concentrations in different concentrations of the water at the same temperature of 60 degrees celsius.
Salt A - 10/ 50 grams H₂OSalt B - 20/60 grams H₂OSalt C - 30/120 grams H₂OSalt D - 40/120 grams H₂OConverting them in the same amount of H₂O that is 100 grams in the same ratio:
Salt A - 10/ 50 = x/100 gram = 20 gram/ 100 gram H₂OSalt B - 20/60 = x/100 grams = 33.3 gram/ 100 gram H₂OSalt C - 30/120 = x/100 grams = 25 gram/ 100 gram H₂OSalt D - 40/120 = x/100 grams = 50 gram/ 100 gram H₂OThus, the salt D is the most soluble that is 50 grams per 100 gram H₂O.
Learn more:
https://brainly.com/question/22185953
please help asap in 10 mins
What are the conditions necessary for electro-chemical corrosion to occur?
Answers
Answer:
Presence of an Electrolyte
Metal Surface
Oxygen or Other Oxidizing Agent
Difference in Potential
Electrochemical Pathway
Explanation: