Answers
Here are some questions on Personal Care services on E2020 are:
A client with a new ileostomy has been home for four days. The HHA is giving the client a bath and notices that the pouch is full. The HHA should say to the client: D. "I'll empty the pouch for you."A client is bedridden at home and has an infected draining sacral wound. The infection control supplies that should be kept in the home is: Gloves.What is infection?An infection is the entrance and growth of dangerous microorganisms in the body that harm the host, such as bacteria, viruses, fungus, or parasites.
Infections can be systemic (affecting the entire body) or localized (affecting a particular area of the body), and they can be moderate to severe.
Learn more about infection on https://brainly.com/question/14083398
#SPJ1
Related Questions
What volume of 1/5 M and 1/8 M HCl solution must be mixed to obtain 3 L of 1/7 M HCl?
Answers
0.72 L and 2.28 L of 1/5 M and 1/8 M HCl solution are required respectively to obtain 3 L of 1/7 M HCl.
What is the concentration of a solution?The concentration of a solution is the amount of substance a given volume of the solution of that substance.
The final concentration and volume of the mixture is 3 L of 1/7 M HCl.
Let the volume of 1/5 M and 1/8 M HCl solution required be X and Y respectively.
X + Y = 3 L
Liters of y = 3 -
[.0.2 X + 0.125 * (3-X)] / 3 = 1/7
(0.2X + 0.375 - 0.125X) / 3 = 1/7
(0.075 X / 3) = 1/7 - 0.375/3
(0.075X / 3) = 0.018
Multiplying both sides by 3
0.075 X = 0.054
X = 0.72 L
Y = 2.28 L
Therefore, 0.72 L and 2.28 L of 1/5 M and 1/8 M HCl solution are required respectively.
Learn more about concentration at: https://brainly.com/question/24595796
Name the alkyne please help!!

Answers
Answer:
4-ethylhex-2-yne
Explanation:
Give and proidi the following after and undergoing alpha decay and beta decay
Answers
The products of the alpha decay of radium-226 and the beta decay of carbon-14 are radon-222 and nitrogen-14, respectively.
The alpha decay of radium-226 results in the emission of an alpha particle, which is a helium nucleus consisting of two protons and two neutrons.
Therefore, the product of the alpha decay of radium-226 is radon-222:
Ra-226 → Rn-222 + alpha particle
On the other hand, In the case of carbon-14, beta minus decay occurs, in which a neutron is converted into a proton, and an electron and an antineutrino are emitted.
So carbon-14 becomes nitrogen-14:
C-14 → N-14 + beta particle
To know more about alpha decay, here
brainly.com/question/27870937
#SPJ1
--The complete Question is, What is the product of the alpha decay of radium-226 and the beta decay of carbon-14?--
The volume measuring system for a gasoline pump at the service station is calibrated at 20.0°C. If the temperature of the gasoline drops to 10.0°C, what percentage extra amount of mass of gasoline do you receive when making a purchase? The coefficient of volume expansion for gasoline is 9.5 × 10-4/K.
Answers
Answer:
answer = -0.95
Explanation:

the freezing point of a 1 m solution of f e c l 3 is expected to be lower than check circle outline that of a 1 m solution of glucose. this is because f e c l 3 is more soluble highlight off and has fewer moles of solute highlight off compared with glucose.
Answers
The freezing point of a 1 m solution of FeCl3 is expected to be lower than that of a 1 m solution of glucose because FeCl3 is more soluble and has more moles of solute compared with glucose.
This is due to the fact that FeCl3 is an ionic compound, which dissociates into Fe3+ and 3 Cl- ions in solution. This means that there are more particles in solution, which lowers the freezing point. Glucose, on the other hand, is a covalent compound and does not dissociate into ions in solution. Therefore, there are fewer particles in solution, which results in a higher freezing point. This phenomenon is explained by the colligative property of freezing point depression, which states that the freezing point of a solution is lowered by the presence of solute particles. The more particles in solution, the lower the freezing point.
More freezing point questions can be found here: https://brainly.com/question/27184888
#SPJ11
The chemical formula for magnesium oxide is 2MgO. The number 2 represents the number of ___.
oxygen atoms
magnesium atoms
magnesium oxide molecules
Answers
Answer:
Magnesium oxide molecules
Explanation:
which of the following statements regarding membranes is true? which of the following statements regarding membranes is true? both faces of membranes tend to have similar compositions. transverse diffusion occurs rapidly. bilayer formation is largely driven by the hydrophobic effect. lateral diffusion is largely dependent on an enzyme-mediated process.
Answers
It is accurate what is said about membranes below: an enzyme-mediated mechanism is mostly responsible for lateral diffusion.
What is the difference between hydrophilic and hydrophobic substances?A substance can be either hydrophobic or hydrophilic. Given that the word "hydr" is derived from the Greek word "hydor," which means "water," hydrophobic materials are "water-fearing" and do not blend with water, whereas hydrodynamic materials are "water-loving" and have a propensity to become wetted by water.
What does hydrophobic substance mean?Non-polar substances with a low affinity for water are referred to as hydrophobic substances and are water-repellent. As opposed to a hydrophobic interaction, which is indicated by a contact angle larger than 90°, a hydrophilic interaction is indicated by a contact angle less than 90°.
To know more about Hydrophobic visit:
https://brainly.com/question/16003692
#SPJ1
For each reaction, write the chemical formulae of the oxidized reactants in the space provided. Write the chemical formulae of the reduced reactants.
reactants oxidized _____
reactants reduced _____
a. 2Fe(s)+3Pb(NO3)2(aq)→3Pb(s)+2Fe(NO3)3(aq)
b. AgNO3(aq)+Cu(s)→2Ag(s)+CuNO)2(a)
c. 3AgNO(aq)+Al()→3Ags)+Al(NO3)3(aq)
Answers
Answer:
a. Oxidized: Fe(s)
Reduced: Pb(NO3)2
b.Oxidized: Cu(s)
Reduced: AgNO3
c. Oxidized: Al(s)
Reduced: AgNO3
Explanation:
In a redox reaction, one reactant is been oxidized whereas the other is reduced.. The reduced reactant is the one that is gaining electrons and the oxidized one is loosing electrons.
In the reactions:
a. 2Fe(s)+3Pb(NO3)2(aq)→3Pb(s)+2Fe(NO3)3(aq)
The Fe is as reactant as Fe(s) (Oxidation state 0) and the product is +3 (Because NO3, nitrate ion, is always -1). That means Fe is oxidized. The Pb as reactant is +2 and as product 0 (Gaining 2 electrons). Pb(NO3)2 is reduced
b. 2AgNO3(aq)+Cu(s)→2Ag(s)+Cu(NO3)2(a)
AgNO3 is +1 and Ag(s) is 0. AgNO3 is reduced. Cu(s) is 0 as reactant and +2 as product. Cu(s) is been oxidized
c. 3AgNO3(aq)+Al(s)→3Ag(s)+Al(NO3)3(aq)
Here, in the same way, AgNO3 is +1 as reactant and 0 as product. AgNO3 is reduced. And Al(s) is 0 as reactant but + 3 as product. Al(s) is oxidized.
If 3.90 g of CuNO3 is dissolved in water to make a 0.840 M solution, what is the volume of the solution in milliliters?
Volume:
Answers
The volume of the solution is 24.8 milliliters (mL).
To solve this problem, we can use the formula:
Molarity (M) = moles of solute / liters of solution
We are given the mass of CuNO3 and the molarity of the solution, so we can first calculate the number of moles of CuNO3:
moles of CuNO3 = mass / molar mass
molar mass of CuNO3 = 63.55 + 14.01 + 3(16.00) = 187.56 g/mol (using atomic weights from the periodic table)
moles of CuNO3 = 3.90 g / 187.56 g/mol = 0.0208 mol
Next, we can rearrange the formula for molarity to solve for the volume of the solution:
liters of solution = moles of solute / Molarity
liters of solution = 0.0208 mol / 0.840 M = 0.0248 L
Finally, we can convert liters to milliliters:
volume of solution = 0.0248 L x 1000 mL/L = 24.8 mL
For more question on volume click on
https://brainly.com/question/27100414
#SPJ11
S + 6 HNO3 --> H₂SO4 + 6 NO2 + 2H₂O
In the above equation how many grams of water can be made when 6 grams of HNO3 are
consumed?
Answers
Taking into account the reaction stoichiometry,50.57 grams of H₂O are formed when 6 grams of HNO₃ are consumed.
Reaction stoichiometryThe balanced reaction is:
S + 6 HNO₃ → H₂SO₄ + 6 NO₂ + 2 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
S: 1 moleHNO₃: 6 molesH₂SO₄: 1 moleNO₂: 6 molesH₂O: 2 molesThe molar mass of the compounds is:
S: 32 g/moleHNO₃: 63 g/moleH₂SO₄: 98 g/moleNO₂: 46 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
S: 1 mole ×32 g/mole= 32 gramsHNO₃: 6 moles ×63 g/mole= 378 gramsH₂SO₄: 1 mole ×98 g/mole= 98 gramsNO₂: 6 moles ×46 g/mole= 276 gramsH₂O: 2 moles× 18 g/mole= 36 gramsMass of H₂O formedThe following rule of three can be applied: if by reaction stoichiometry 378 grams of HNO₃ form 36 grams of H₂O, 6 grams of HNO₃ form how much mass of H₂O?
mass of H₂O= (6 grams of HNO₃×36 grams of H₂O)÷378 grams of HNO₃
mass of H₂O= 0.57 grams
Finally, 50.57 grams of H₂O are made.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
what happens to a circuits resistance (R), voltage (V), and current (I) when you increase the diameter of the wire in the circuit?
a. R increases, V is constant, I increases
b. R decreases, V is constant, I increases
c. R decreases, V increases, I increases
d. R increases, V decreases, I decreases
Answers
Answer:
b. R decreases, V is constant, I increases
Explanation:
when we increase the diameter of wire increases ,resistance decreases and current increases.Therefore, the option is b.
Resistance is an electrical quantity which opposes the electric current in the circuit. Resistance is directly proportional to the length of the wire and inversely proportional to the diameter of the wire. If length of wire increases resistance will increases and if the diameter of the wire increase resistance will decreases.
R = ρ L/A
Here ρ is the resistivity
L is the length of the wire
A is the cross-sectional area of wire/diameter of the wire
Voltage is the potential difference between two terminals of voltage source. Current is the flow of electrons in the circuit. Voltage is the product of current and resistance.
V=IR
Rewrite the above equation interms of current
I=V/R
From the above equation we can say that current is inversly proportional to the resistance.Therefore,the correct option is b.
Learn more about,resistance
https://brainly.com/question/4289257
Which statement defines dynamic equilibrium? A state of balance in which the rates of the forward and reverse reactions are equal. A state of balance in which the forward reaction stops but reverse reaction continues. A state of balance in which the forward reaction continues but reverse reaction stops. A state of balance in which the forward and reverse reactions stop.
Answers
Answer:
A state of balance in which the rates of the forward and reverse reactions are equal.
Explanation:
The dynamic equilibrium is a state of balance in which the rates of the forward and reverse reactions are equal, like we can see in the following drawing:
And the concentration of the reactants and products become stable in time.

Answer: A state of balance in which the rates of the forward and reverse reactions are equal.
Explanation:
chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time.
A student conducted three trails to determine the concentration of an unknown concentration of HCI. In the first trail the calculated concentration was 0.104 M, the second was 0.113 M and the third trail was 0.108 M. What is the percent difference between the first two trials and based on the lab procedures procedures guidelines what would the average molarity be?
Answers
The percent difference between the first two trials is 8.3% and final outcome would be an average molarity of 0.108 M.
How to calculate percent difference and average molarity?To calculate the percent difference between the first two trials, use the formula:
% Difference = |(Value 1 - Value 2) / ((Value 1 + Value 2) / 2)| x 100%
% Difference = |(0.104 M - 0.113 M) / ((0.104 M + 0.113 M) / 2)| x 100%
% Difference = |-0.009 M / 0.1085 M| x 100%
% Difference = 8.3%
The percent difference between the first two trials is 8.3%.
To find the average molarity, add the three calculated concentrations together and divide by the number of trials:
Average Molarity = (0.104 M + 0.113 M + 0.108 M) / 3
Average Molarity = 0.108 M
Based on the lab procedures guidelines, the average molarity would be the most accurate representation of the unknown concentration of HCI. Therefore, the average molarity of 0.108 M would be the final result.
Find out more on percent difference here: https://brainly.com/question/10791047
#SPJ1
If a neutral atom has 17 electrons, what type of ion will it tend to form?
A. cation with charge 1+
B. anion with charge 2-
C. anion with charge 1-
D. cation with charge 1-
Answers
Answer:
C. anion with charge 1-
Explanation:
Atoms of group 17 gain one electron and form anions with a 1− charge; atoms of group 16electrons and form ions with a 2− charge, and so on.
If a neutral atom has 17 electrons, what type of ion will it tend to form anion with charge 1-. Thus option C is correct.
What are the properties of atom ?
Matter is defined as anything which takes space and mass called as Matter and these matter is made up of atom or any other element that make up of space and mass only.
Atoms are composed of sub atoms like positively charged proton, neutral neutron, negatively charged electron which are bond together; proton and neutron present in nucleus at the center of the atom where as electron surround them.
A number of atoms are joined to form molecule, which are present in three form such as solid, liquid and gas, we cannot break atom which is the smallest unit of a matter.
A compound is a molecule which are made up of number of atoms by forming a chemical bond, the bond may be covalent bond, ionic bond, metallic bonds, coordinate covalent bonds.
Atoms of group 17 which gain one electron and it form anions with a 1− charge while atoms with group 16 electrons and form ions with a 2− charge. Thus option C is correct.
Learn more about atom, here:
https://brainly.com/question/28362713
#SPJ2
How many moles of a gas sample are in a 5.0 L container at 373 K and 203 kPa?
0.33 mole
0.66 mole
1.11 moles
3.05 moles
Answers
Answer:
0.33 moles.
Explanation:
Assuming the gas is ideal, we can solve this problem by using the following formula:
PV = nRTWhere:
P = 203 kPaV = 5.0 Ln = ?R = 8.314 kPa·L·mol⁻¹·K⁻¹T = 373 KWe input the data:
203 kPa * 5.0 L = n * 8.314 kPa·L·mol⁻¹·K⁻¹ * 373 Kn = 0.327 molThe answer is thus the first option, 0.33 moles.
Answer:
0.33 mole
Explanation:
A nurse practitioner prepares 470. mL of an IV of normal saline solution to be delivered at a rate of 85 mL/h. What is the infusion time, in hours, to deliver 470. mL?
Answers
Answer: Infusion time is 5.5 h
Explanation: Time is 470 ml / 85 ml/h = 5.5 h
Can anyone help me understand how to calculate the moles of H+ and OH-?

Answers
To calculate the moles of H+ and OH-, you need to know the concentration of the solution in terms of its pH or pOH value.
How to calculate the molesWhen you get the pH of the solution, you can use this formula to calculate the concentration of H+ ions: [H+] = 10^(-pH)
Also, if you know the pOH of the solution, you can use this formula to calculate the concentration of OH- ions: [OH-] = 10^(-pOH)
Having determined the concentration of H+ and OH- ions, the molarity formula can be used to calculate the number of moles of each ion as follows: moles = concentration (in mol/L) x volume (in L)
Learn more about moles calculation here:
https://brainly.com/question/14357742
#SPJ1
A: Light
B: Sound
C: Heat
D: Motion
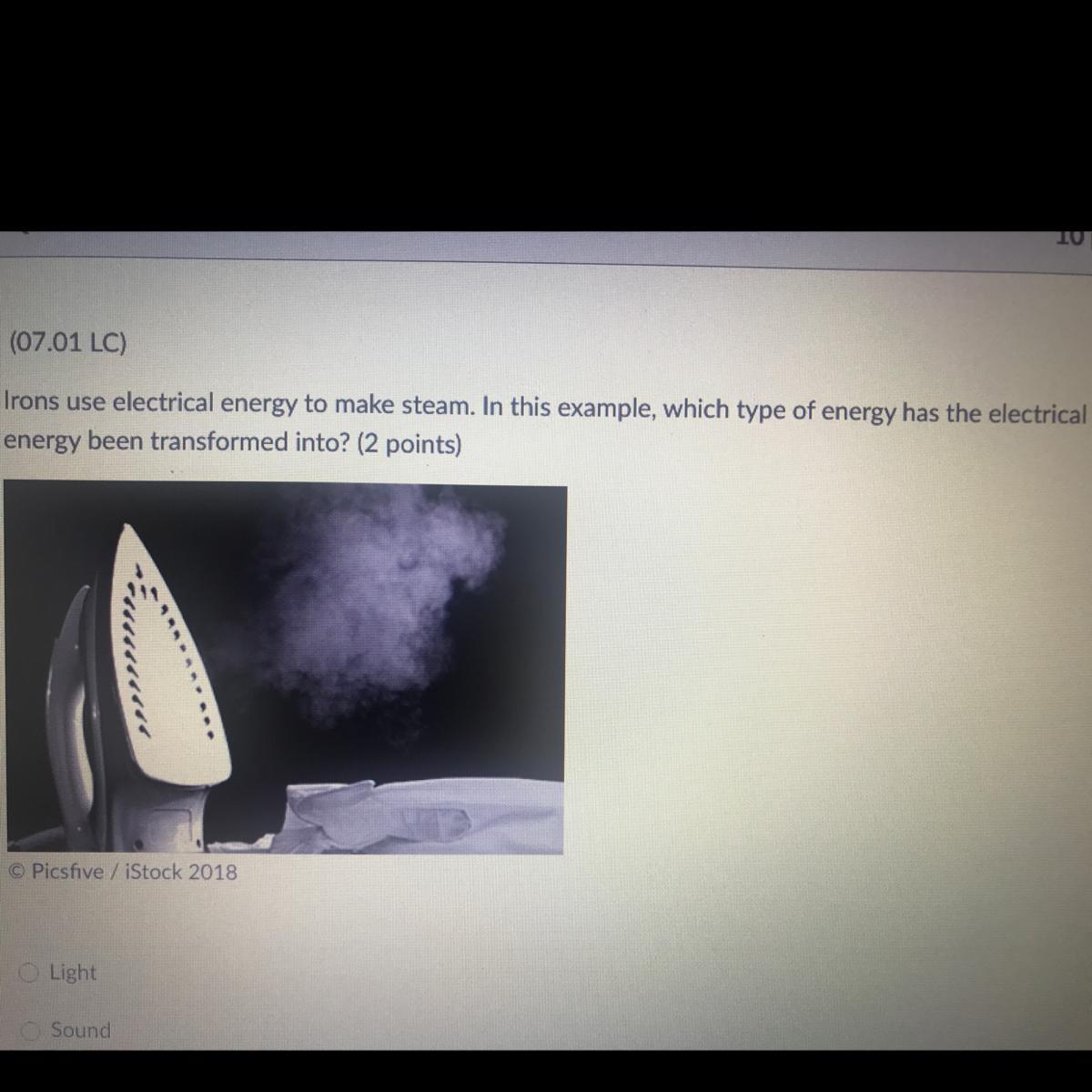
Answers
A basketball is inflated to 9.5 psi above atmospheric pressure of 14.7 psi, for a total pressure of 24.2 psi. The diameter of the basketball is 9.39inches. a.) What is the pressure of the air in the basketball in atmospheres? b.) what is the volume of the basketball in liters? The volume of a sphere is = (4/3)pi(r)^3
Answers
Answer:
a. 21.8 psi
b. 17.32 Liters
Explanation:
Sofia goes on a hike on a trail that is 10 km long. She starts at 2:00pm and ends at
5:00pm. The end of the trail is 300m north of the beginning of the trail. What is
Sofia's average velocity?
(Velocity= displacement/time)
100 m/hr S
30 m/hrs
0.01 m/hrs
3.3 m/hr s

Answers
Answer:
100 m/hr S
Explanation:
Use formula Velocity = displacement/time
V=? d=300 t=3
V=300/3
V=100
Hope this helps!! :D
Sofia's average velocity is 100 m/hrs using the velocity formula which is displacement/time.
What is average velocity?Speed of an object refers to the change in position of that object concerning time. Velocity on the other hand is nothing, but the speed defined concerning the direction in which an object travels.
Average velocity according to the definition is the ratio of the displacement from point a to point b of an object to the time it takes to make that displacement from point a to point b. It may be noted that we use the term movement instead of distance to emphasise the direction.
Velocity is a vector quantity and average velocity can be found by dividing displacement by time. It is found out as, 300/3=100 m/hrs.
Thus, Sofia's average velocity is 100 m/hrs using the velocity formula which is displacement/time.
Learn more about average velocity,here:
https://brainly.com/question/862972
#SPJ2
Does H form cation, anion both or neither ions?
Answers
Answer:
hydrogen have electronic configuration of 1s¹ to acquire a stable state it can either lose electron and form H+cation or gain an electron( to compete its 1s subshell ) to form H- anion. As it has ±1 valency it is placed neither in group 1(alkaline metals ) nor in group 17 (halogens) .Which of the following substances did Robert Brown observed upon forming the concept of Brownian motion?
A. Talc powder suspended in water
B. Talc powder suspended in air
C. Pollen grains suspended in water
D. Pollen grains suspended in air
Answers
Answer:
Talc powder suspended in air
Q25 Apply the Criss-Cross method to determine formulae of the corresponding salt's with
two different examples?
Answers
Answer:
CaCl2
CaO
Explanation:
Ca^2+ 2Cl^-
Ca^2+ O^2-
Which example is a biotic factor of an aquarium environment?
Responses
amount of oxygen in the water
water temperature
amount of sand in the aquarium
number of underwater plants
Answers
To solve this we must be knowing each and every concept related to environment. Therefore, the correct option is option B among all the given options.
What is environment?An environment may be simply defined as a system that includes all abiotic and biotic components that have an impact on human life. All flora and animals are considered biotic, or living, elements, whereas water, sunshine, air, temperature, etc. are considered abiotic.
Any good, service, or feature that benefits people and society might be considered one of an environment's resources. They might be anything that fulfills a person's requirements on a daily basis. Amount of oxygen in the water is a biotic factor of an aquarium environment.
Therefore, the correct option is option B.
To know more about environment, here:
https://brainly.com/question/28962722
#SPJ1
Answer: B: Water Temperature
Explanation: k12 test 1.04 Science Life semester 2
Explain ocean currents and how density differences between HOT/COLD and SALT/FRESH affect them.
-for science
Answers
Density differences caused by temperature and salinity variations are fundamental drivers of ocean currents of seawater . Warm currents transport heat from the equator to higher latitudes, while cold currents transport cold water from higher latitudes to lower latitudes.
When seawater is heated, it expands and becomes less dense, causing it to rise. Conversely, when seawater cools, it contracts and becomes denser, causing it to sink. These density differences due to temperature variations create vertical movements in the ocean known as thermohaline circulation or convection currents. On the other hand, regions with high freshwater input from rivers or heavy precipitation have lower salinity, resulting in lower density. This lighter water tends to float on the denser seawater beneath it, leading to the formation of surface currents that transport water from areas of low salinity to areas of higher salinity. These ocean currents play a vital role in shaping global climate patterns and maintaining the balance of heat and nutrients in the ocean ecosystem.
Learn more about the ocean current here.
https://brainly.com/question/21654036
#SPJ1
list three statements for transverse waves
Answers
•Transverse waves are a form of traveling energy
•Transverse waves have oscillations perpendicular to the direction of energy transfer
•Transverse waves have particles that move about their fixed position
Will give brainliest!
The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl + Ca(NO3)2
How many grams of AgCl are produced from 30.0 grams of CaCl2?
(Molar mass of Ca = 40.078 g/mol, Cl = 35.453 g/mol, O = 15.999 g/mol, Ag = 107.868 g/mol, N = 14.007 g/mol)
A. 19.4 grams
B. 38.8 grams
C. 58.2 grams
D. 77.5 grams
Answers
Explanation:
Molar mass of CaCl2 = 110.98g/mol
Moles of CaCl2 = 30.0 / 110.98 = 0.270mol
Moles of AgCl = 0.270mol * 2 = 0.540mol
Mass of AgCl = 0.540mol * (143.32g/mol) = 77.39g.
Answer:
77.5
Explanation:
You gotta round.
Hope this helps :)
characteristics. of. rusting
Answers
Answer: metal turn orange and weaker as it gets oxidised
Explanation:
How are solutions and compounds similar?
Answers
Answer:
hope you liked it!!!!!!
A compound is a pure substance that is composed of elements chemically bonded in definite proportions. A compound can be broken down into simpler substances only by chemical reactions, such as electrolysis.
A solution is a homogeneous mixture, meaning that it is the same throughout. A solution is composed of one or more solutes dissolved in a solvent. The proportions of the solute(s) can vary, as the components of a solution are not chemically bonded. The components of a mixture can be separated by physical means, such as filtration and distillation
Why does the solubility of many substances increase with temperature? (Remember what an increase in temperature means on a microscopic scale.)
Answers
The solubility of many substances increases with temperature, there are exceptions. Some substances exhibit a decrease in solubility with temperature due to specific interactions or changes in solute-solvent interactions at higher temperatures.
The increase in solubility of many substances with temperature can be attributed to the effect of temperature on the kinetic energy and intermolecular interactions of molecules.
On a microscopic scale, an increase in temperature corresponds to an increase in the kinetic energy of molecules. As the kinetic energy increases, the molecules move more rapidly and collide with each other and with the solvent molecules more frequently and with greater force.
These increased collisions and kinetic energy result in enhanced molecular interactions and overcome the forces holding the solute particles together. This increased energy disrupts the intermolecular forces within the solute, allowing the solvent molecules to surround and interact more effectively with the solute particles, leading to greater solubility.
Additionally, an increase in temperature can cause solvent molecules to move more freely, reducing their cohesion and allowing them to interact more readily with solute particles.
for more questions on solubility
https://brainly.com/question/24057916
#SPJ8