Answers
Answer: The African savanna ecosystem is a tropical grassland with warm temperatures year-round and seasonal rainfall. The savanna is characterized by grasses and small or dispersed trees, along with a diverse community of organisms that interact to form a complex food web.
Related Questions
Name the following lonic Compounds using the lonic naming rules. Remember, place the metal's name
first, followed by the non-metal element, replacing the ending with "-ide"
1.Caci,
2.LIBr
I
3. Bes
4. LIF
5. K Se
6. Sr,P2
7. Baci
8. Feo
9. Fe,
10. CUN
11. Cun,
Please help meeee
Answers
2.Lithium Bromide
3.Beryllium Sulfide
4.Lithium Fluoride
5. Potassium hydroselenide
6. Strontium phosphide
7.Barium Chloride
8.Iron Oxide
9.Iron
10.?
11.Copper Nitride
6.45g of lithium reacts with 9.2g of oxygen gas to produce lithium oxide.How many grams of Li20 are formed? Hint: write the balanced chemical equation first.
Answers
Stoichiometry:
Choose a given first.
6.45g Li • 1 mole Li 2 moles Li2O 30 g. Li2O
————- • ———- • ——
7 g Li 4 moles Li** 1 mol. Li2O
** Mole to mole ratio from balanced equation
Multiple everything on top and divide by everything multiplied on bottom.
387
———- = ~ 13.8 g. Li2O
28
(Al = 27.0 g, O = 16.0 g, H = 1.0 g)
2 Al(OH)3 Al2O3 + 3 H2O
how many grams are produced from .85 moles of AI(OH)3
Answers
Answer:
Explanation:
21
HELPPP PLEASEE w/ all

Answers
The covalent bond is present in the compound C₃H₈. The reactant C is 3, product C is 6, reactant H is 8, product H is 10, Reactant O is 2, product O is 9.
What is covalent bond ?
Atoms share electron pair between them in covalent bonds. H-H or C-H are examples of nonpolar covalent bonds between atoms with similar or identical electronegativity, whereas polar covalent bonds are formed when unequal electronegativity is shared between atoms (e.g., H–O).
What is reactant ?
Raw materials known as reactants combine to create products. When the right factors, such as temperature, time, or pressure, come into play, the chemical bonds between the reactants are broken, allowing the atoms to form new bonds that lead to various combinations.
Therefore, covalent bond is present in the compound C₃H₈. The reactant C is 3, product C is 6, reactant H is 8, product H is 10, Reactant O is 2, product O is 9.
Learn more about covalent bond from the given link.
https://brainly.com/question/3447218
#SPJ1
Arrange the compounds by their reactivity toward electrophilic aromatic substitution.
a. Benzene, ethylbenzene, chlorobenzene, nitrobenzene, anisole.
b. Toluene, p-cresol, benzene, p-xylene.
c. Benzene, benzoic acid, phenol, propylbenzene.
d. p-Methylnitrobenzene, 2-chloro-1-methyl-4-nitrobenzene, 1-methyl-2,4-dinitrobenzene, p-chloromethylbenzene.
Answers
Answer:
The order of reactivity towards electrophilic susbtitution is shown below:
a. anisole > ethylbenzene>benzene>chlorobenzene>nitrobenzene
b. p-cresol>p-xylene>toluene>benzene
c.Phenol>propylbenzene>benzene>benzoic acid
d.p-chloromethylbenzene>p-methylnitrobenzene> 2-chloro-1-methyl-4-nitrobenzene> 1-methyl-2,4-dinitrobenzene
Explanation:
Electron donating groups favor the electrophilic substitution reactions at ortho and para positions of the benzene ring.
For example: -OH, -OCH3, -NH2, Alkyl groups favor electrophilic aromatic substitution in benzene.
The -I (negative inductive effect) groups, electron-withdrawing groups deactivate the benzene ring towards electrophilic aromatic substitution.
Examples: -NO2, -SO3H, halide groups, Carboxylic acid groups, carbonyl gropus.
Which area of science is considered the central science?
chemistry
biology
physics
environmental science
Answers
Answer:
A. Chemistry.
Explanation:
A saturated solution of sucrose in 1000.0 g of boiling water is cooled to 20°C.
What mass of rock candy will be formed?
(Keep in mind that it’s solubility at 20° is 230.9)
Answers
No rock candy will be formed, as the solution is already at its maximum saturation point at 20°C.
We can calculate the maximum amount of sucrose that can be dissolved in 1000.0 g of water at this temperature as follows:
The maximum amount of sucrose that can be dissolved = \((230.9 g/100 mL) * (1000.0 mL/1000.0 g) = 2.309 g/g\)
This means that the maximum mass of sucrose that can be dissolved in 1000.0 g of water at 20°C is 2.309 g.
Mass of rock candy = Initial mass of sucrose - Mass of sucrose in solution at 20°C
Therefore, the mass of rock candy that will be formed is:
Mass of rock candy = Initial mass of sucrose - Mass of sucrose in solution at 20°C
\(= 2.309 g - 2.309 g/g * 1.000 kg \\= 0.0 g\)
To know more about saturation point, here
brainly.com/question/14118324
#SPJ1
Which of the following is the Arrhenius Theory of acids and bases? O An acid dissociates in water to form Hydrogen ions (H^+) and a base dissociates in water to produce Hydroxide ions (OH^-). O An acid is a proton donor and a base is a proton acceptor. O Acids are substances with a very high pH (greater than 10) and bases are substances with a very low pH (less than 3). O None of the above.
Answers
In water, a highly soluble sodium hydroxide compound dissociates to give sodium ion and hydroxide ion as an example of an Arrhenius base.
To enhance the concentration of hydroxide ions, NaOH entirely dissolves in aqueous solution to give hydroxide ion and sodium ion. The Arrhenius theory, proposed in 1887 by the Swedish scientist Svante Arrhenius, states that acids dissolve in water to produce electrically charged atoms or molecules known as ions, one of which is a hydrogen ion (H+), and that bases ionize in water to produce hydroxide ions (OH). As a result, water is a material that dissociates in water to create H+ ions. It also meets the definition of a material that dissociates in water to create OH ions.
Learn more about hydroxide here-
https://brainly.com/question/4251554
#SPJ4
Ascorbic acid (H2C6H6O6), also known as Vitamin C, is a polyprotic acid found in fruit, tomatoes, potatoes and leafy vegetables. The pKa's of the ascorbic acid are pKa1 = 4.10 and pKa2 = 11.80 at 25 °C. When ascorbic acid is titrated with NaOH and it takes 60.0 mL to remove all protons possible. In the titration curve, how many mL of NaOH are required to reach the first pKa? Another way of asking this is when presented with a titration curve of ascorbic acid where on the x-axis should one look (which volume of NaOH), if one wants to determine the first pKa?
Answers
Answer:
as when we add 60 ml it removes all the protons it means it is 2nd equivalence point of Ascorbic acid
and we know that pH = pKa1 when moles of NaOH is half of the 1st equivalence point
1st equivalence point = 2nd equivalence point / 2 = 60/2 =30ml
1st half equivalence point = 30/2 = 15ml
so when we add 15ml of NaOH
pH = pKa1 =15mL
If the experiment were repeated using a cuvettes with a path length of 2.0 cm, how would that affect the absorbance values for the Co2 solutions (if at all), assuming all of the concentrations remained unchanged?
Answers
Answer:
..............................
Insoluble sulfide compounds are generally black in color. Which of the following combinations could yield a black precipitate? a) Na2S(aq) + KCl(aq) b) Li2S(aq) + Pb(N03)2(aq) c) Pb(C103)2(aq) + NaNO3(aq) d) AgNo3(aq) + KCl(aq) e) K2S(aq) + Sn(N03)4(aq)
Answers
Answer:
b) Li2S(aq)+Pb(NO3)2(aq)
e) K2S(aq)+Sn(NO3)4(aq)
Explanation:
they are the only two of the options that contain a sulfide ion (S) therefore they are the only ones that could be considered in the question.
b) Li2S(aq)+Pb(NO3)2(aq)
e) K2S(aq)+Sn(NO3)4(aq)
A solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO3)2(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 16.83g PbCl2(s) is obtained from 200.0mL of the original solution. Calculate the molarity of the Pb(NO3)2(aq) solution.
Answers
0.25 Molarity of lead nitrate aqueous solution is there when a solution of nacl is added slowly to a solution of lead nitrate.
What is molarity and how it is calculated out to be so?Molarity is the number of moles of substance present in one litre of solution and is measured in g/litres.Here in this question is given 16.83 g of lead nitrate is obtained from 200 ml of original solution.To calculate the molarity first we will have to calculate the number of moles of lead nitrate for which the formula is given mass/ molar mass.The mass that is given is 16.83 g and the molar mass is 331.2 g dividing we will get 5.02.Then dividing the number of moles by 0.2 L of solution we will get the answer as 0.25 g/litre molarity as the answer.To know more about molarity visit:
https://brainly.com/question/8732513
#SPJ10
A gas phase mixture of H2 and N2 has a total pressure of 784 torr with an H2 partial pressure of 124 torr. What mass of N2 gas is present in 2.00 L of the mixture at 298 K
Answers
The mass of N₂ in the mixture having a total pressure of 784 torr with an H₂ partial pressure of 124 torr is 1.988 g
We'll begin by calculating the partial pressure of N₂. This can be obtained as follow:
Total pressure = 784 torr
Partial pressure of H₂ = 124 torr
Partial pressure of N₂ =?Total pressure = Partial pressure of H₂ + Partial pressure of N₂
784 = 124 + Partial pressure of N₂
Collect like terms
Partial pressure of N₂ = 784 – 124
Partial pressure of N₂ = 660 TorrNext, we shall determine the number of mole of N₂ in the mixture.Pressure of N₂ (P) = 660 Torr
Volume (V) = 2 L
Temperature (T) = 298 K
Gas constant (R) = 62.364 L.Torr/Kmol
Number of mole of N₂ (n) =?PV = nRT
660 × 2 = n × 62.364 × 298
1320 = n × 18584.472
Divide both side by 18584.472
n = 1320 / 18584.472
n = 0.071 moleFinally, we shall determine the mass of N₂ in the mixture.Mole of N₂ = 0.071 mole
Molar mass of N₂ = 2 × 14 = 28 g/mol
Mass of N₂ =?Mass = mole × molar mass
Mass of N₂ = 0.071 × 28
Mass of N₂ = 1.988 gTherefore, the mass of N₂ in the mixture having a total pressure of 784 torr with an H₂ partial pressure of 124 torr is 1.988 g
Learn more: https://brainly.com/question/20853110
Determine the concentrations of BaBr2, Ba2+, and Br− in a solution prepared by dissolving 2.38 × 10−4 g BaBr2 in 2.00 L of water. Express all three concentrations in molarity. Additionally, express the concentrations of the ionic species in parts per million (ppm).
Answers
The BaBr₂ will dissolve in water and form its ions according to this equation:
BaBr₂ ----> Ba²⁺ + 2 Br⁻
We have to find the molarity of the solution. The molarity of a solution is calculated using this formula:
Molarity = moles of solute / volume of solution in L
We know the volume of solution (2.00 L) and we know the mass of BaBr₂. To find the molarity of the solution we will have to convert those grams into moles. When we have to convert a mass into moles we use the molar mass. Let's start finding the molar mass of BaBr₂.
atomic mass of Ba: 137.33 amu
atomic mass of Br: 79.90 amu
molar mass of BaBr₂ = 137.33 + 2 * 79.90
molar mass of BaBr₂ = 297.13 g/mol
Now we can find the number of moles of BaBr₂ that we have in 2.38 *10^(-4) g of it.
moles of BaBr₂ = 2.38 *10⁻⁴ g / (297.13 g/mol)
moles of BaBr₂ = 8.01 *10⁻⁷ moles
So we prepared a solution dissolving 8.01 * 10⁻⁷ moles of BaBr₂ in 2.00 L of water. Let's find the molarity:
Molarity of BaBr₂ = moles of BaBr₂ / volume of solution in L
Molarity of BaBr₂ = 8.01 * 10⁻⁷ moles/(2.00 L)
Molarity of BaBr₂ = 4.00 * 10⁻⁷ M
We said that the BaBr₂ will dissolve in water according to this equation:
BaBr₂ ----> Ba²⁺ + 2 Br⁻
If we look at the coefficients we see that 1 mol of BaBr₂ will produce 1 mol of Ba²⁺ ions and 2 moles of Br⁻ ions.
So we can say that:
moles of Ba²⁺ = moles of BaBr₂
moles of Ba²⁺ = 8.01 * 10⁻⁷ moles
moles of Br⁻ = 2 * moles of BaBr₂
moles of Br⁻ = 2 * 8.01 * 10⁻⁷ moles
moles of Br⁻ = 1.60 * 10⁻⁶ moles
Now we can find the molarity of each ion:
Molarity of Ba²⁺ = moles of Ba²⁺ / volume of solution in L
Molarity of Ba²⁺ = 8.01 * 10⁻⁷ moles/(2.00 L)
Molarity of Ba²⁺ = 4.00 * 10⁻⁷ M
Molarity of Br⁻ = moles of Br⁻ / volume of solution in L
Molarity of Br⁻ = 1.60 * 10⁻⁶ moles / (2.00 L)
Molarity of Br⁻ = 8.00 * 10⁻⁷ M
Answer: The concentration of BaBr₂ and the concentration of Ba²⁺ is 4.00 * 10⁻⁷ M. The concentration of Br⁻ is 8.00 * 10⁻⁷ M.
When we express the concentration in ppm we want the concentration in mg/L. We have the concentration in M (moles/L), so to find the concentration in ppm we have to convert moles to mg.
The molar masses of the ions are:
molar mass of Ba²⁺ = 137.33 g/mol
molar mass of Br⁻ = 79.90 g/mol
Let's use them to find the concentration in ppm.
concentration of Ba²⁺ = 4.00 * 10⁻⁷ M = 4.00 * 10⁻⁷ moles/L * 137.33 g/mol
concentration of Ba²⁺ = 5.49 * 10⁻⁵ g/L *1000 mg/g
concentration of Ba²⁺ = 0.0549 mg/L = 0.0549 ppm
concentration of Ba²⁺ = 0.0549 ppm
concentration of Br⁻ = 8.00 * 10⁻⁷ M = 8.00 * 10⁻⁷ moles/L * 79.90 g/mol
concentration of Br⁻ = 6.39 * 10⁻⁵ g/L * 1000 mg/g
concentration of Br⁻ = 0.0639 mg/L = 0.0639 ppm
concentration of Br⁻ = 0.0639 ppm
Answer: the concentration of the ion Ba²⁺ is 0.0549 ppm and the concentration of the ion Br⁻ is 0.0639 ppm.
g The chemicals above are dissolved in water to make a solution. Which ones will conduct electricity
Answers
Answer:
This question is incomplete
Explanation:
Although, the question above is incomplete, however the correct option can be deduced from the explanation below.
Compounds/chemicals that dissolve in water to conduct electricity are Ionic/electrovalent compounds. Ionic compounds/salts are compounds that are held together by ionic/electrovalent bond. These compounds dissociate in water to form ions; the dissociated ions formed are the carriers of electric charges hence enabling the salt solution conduct electricity. Examples of these electrovalent/ionic compounds include NaCl, CaCl₂, CsF and MgO.
NOTE: Identify the ionic/electrovalent compounds in the options (from the completed question) to get your answer.
1.)An object falls through the air, gaining speed as it falls. A student claims that this creates new energy, and so it breaks the law of
conservation of energy. Which statement describes why the student is incorrect? (1 point)
A.)Energy is converted from other forms, not created.
B.)Speed is not related to energy in any way.
C.)Energy can be created without breaking the law of conservation of energy.
D.)Equal and opposite amounts of other energy are also created.
2.) A person throws a ball up into the air, and the ball falls back toward Earth. At which point would the kinetic energy be the lowest?
A.) at a point before the ball hits the ground
B.) when the ball is at its highest point
C.) at a point when the ball is still rising
D.) when the ball leaves the persons hand
(3 is a picture )

Answers
Based on the law of conservation of energy, the student is incorrect because energy is converted from other forms, not created.
When a person throws a ball up into the air, and the ball falls back toward Earth, the speed is lowest when the ball leaves the persons hand.
What is the law of conservation of energy?The law of conservation of energy states that energy is neither created nor destroyed but can be transformed from one form to another.
Based on the law of conservation of energy, the student is incorrect because Energy is converted from other forms, not created.
When an object is falling freely under gravity, it's speed is minimum is lowest when the object leaves the persons hand and is maximum just before the object hits the ground.
Therefore, when a person throws a ball up into the air, and the ball falls back toward Earth, the speed is lowest when the ball leaves the persons hand.
Learn more about about conservation of energy at: https://brainly.com/question/166559
5. Where would you find the focus of an earthquake?
Earth Science .
Answers
Answer:
below the surface
Explanation:
Below are the reduction half reactions for chemolithoautotrophic denitrification, where hydrogen is a source of electrons and energy and nitrate is the terminal electron acceptor.
NO3- + 10e- -> N2 (E0 = +0.74 V)
H+ + 2e- -> H2 (E0 = -0.42 V)
If you balance and combine the reactions so that 145 molecules of H2 gas are oxidized to H+, how many molecules of N2 gas will be produced??
Below are the reduction half reactions for chemolithoautotrophic denitrification, where hydrogen is a source of electrons and energy and nitrate is the terminal electron acceptor.
NO3- + 10e- -> N2 (E0 = +0.74 V)
H+ + 2e- -> H2 (E0 = -0.42 V)
If you balance and combine the reactions so that 200 molecules of H2 gas are oxidized to H+, how many electrons will be transferred from hydrogen to nitrogen?
Below are the half reactions for sulfate reduction using acetate as a source of electrons, energy, and carbon.
CO2 + 8e- -> CH3COO- (-0.29 volts)
SO42- + 8e- -> H2S (-0.22 volts)
If you balance and combine the reactions so that 48 molecules of CH3COO- are oxidized to CO2, how many molecules of water will be produced?
Answers
Balance the half-reactions to guarantee that the number of electrons transferred in both reactions is the same:
NO3- + 8H+ + 10e- N2 + 4H2O H2 2H+ + 2e- NO3- + 8H+ + 10e-
The next step is to figure out how many electrons are moved when 145 molecules of H2 are oxidized. We can see from the balanced equation for the H2 half-reaction that 1 molecule of H2 makes 2 electrons:
2H+ + 2e- → H2
As a result, 145 molecules of H2 will yield:
145 molecular units H2 has two protons per molecule. 290 electrons Equals H2
Finally, we can use the denitrification reaction balanced equation to calculate how many molecules of N2 are created for 290 electrons:
1 molecule of N2 is produced by 10 electrons.
Therefore, 1 molecule N2 290 electrons/10 electrons = 29 molecules N2
29 molecules of N2 gas will be created.
To answer the second query, we must balance the half-reactions:
SO42- + 8H+ + 8e- H2S + 4H2O CO2 + 8H+ + 8e- CH3COO- + 2H2O SO42- + 8H+ + 8e- H2S + 4H2O
The balanced formulae show that 8 electrons are transferred in both half-reactions. As a result, in order to oxidize 48 molecules of CH3COO-, 6 molecules of SO42- must be reduced:
There are 48 nuclei CH3COO- 8 electrons per atom 384 protons = CH3CO-
384 electrons (eight electrons per atom) 48 units of SO42- SO42-
As a result, 48 molecules of CH3COO- reduced to CO2 will yield:
six electrons 4 H2O/molecule SO42- 24 units of SO42- H2O
The answer is that 24 molecules of water will be created.
Learn more Reduction reactions
https://brainly.com/question/19528268
#SPJ4
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
What is fire and how does it work
Answers
Answer:
Fire is the result of applying enough heat to a fuel source, when you've got a whole lot of oxygen around. As the atoms in the fuel heat up, they begin to vibrate until they break free of the bonds holding them together and are released as volatile gases. These gases react with oxygen in the surrounding atmosphere.
Explanation:
The basis of the VSEPR model of molecular bonding is __________. Question 1 options: atomic orbitals of the bonding atoms must overlap for a bond to form regions of electron density in the valence shell of an atom will arrange themselves so as to maximize overlap electron domains in the valence shell of an atom will arrange themselves so as to minimize repulsions regions of electron density on an atom will organize themselves so as to maximize s-character Question 2 (1 point)
Answers
Answer:
Electron domains in the valence shell of an atom will arrange themselves so as to minimize repulsions
Explanation:
In accordance with VSEPR theory, the electron domains or electron pairs on the valence shell of the central atom in a molecule always position themselves as far apart in space as possible in order to minimize repulsions.
Recall that according to VSEPR theory, the shape of a molecule depends on the number of electron pairs or electron domains on the valence shell of the central atom of the molecule, both lone pairs and bond pairs. Lone pairs are known to cause more repulsions than bond pairs.
A geologist studies rocks. Which of Earth's spheres would a geologist be most interested in?
Answers
Answer:
Lithosphere
Explanation:
Answer:
ether the crust or lithosphere
Explanation:
exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. what is the specific heat capacity of the metal
Answers
Exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. The specific heat capacity of the metal is 5.984 J/g°C.
What is specific heat capacity?The heat capacity of a sample of a substance divided by the mass of the sample yields the specific heat capacity (symbol c), also known as massic heat capacity. Informally, it is the quantity of heat that must be added to one unit of a substance's mass in order to raise its temperature by one unit. The specific heat capacity unit in the SI is the joule per kelvin per kilogram, or Jkg⁻¹K⁻¹. For instance, the specific heat capacity of water is 4184 J kg⁻¹K⁻¹, or the amount of energy needed to raise 1 kilogram of water by 1 K.
The specific heat capacity of the metal can be calculated using the equation Q = m × c ×ΔT.
Q = 149.6J
m = 10.0g
ΔT = (final Temperature - initial Temperature) = (25°C - 0°C) = 25°C
Plugging these values into the equation, we get:
149.6J = 10.0g × c ×25°C
Solving for c, we get:
c = \(\frac{149.6J}{(10.0g *25C)}\)
c = 5.984 J/g°C
Therefore, the specific heat capacity of the metal is 5.984 J/g°C.
To know more about specific heat capacity, visit:
https://brainly.com/question/29766819
#SPJ1
Oh no! Billy forgot that his calorimeter should have 2 coffee cups to contain all the heat. If Billy dropped a 25.0 g piece of solid iron (Cs= 0.449 J/g°C) at 398 K in a coffee cup containing 25.0 mL of water at 298K and because of his mistake, 21% of the heat that should be transferredfrom the iron to the wateris lost to the atmosphere, calculate the final temperature of the iron water mixture?
Answers
Answer:
Explanation:
Let the final temperature be T .
heat lost by iron = mass x specific heat x decrease in temperature
= 25 x .449 x ( 398 - T )
Heat applied to increase the temperature of water
= 25 x .449 x ( 398- T ) x .79 [ 21 % is lost ]
= 8.86775 ( 398 - T )
heat gained by water = 25 x 4.2 x ( T - 298 )
Both of them are equal
8.86775 ( 398 - T ) = 25 x 4.2 x ( T - 298 )
8.86775 ( 398 - T ) = 105 x ( T - 298 )
105 T + 8.86775 T = 105 x 298 + 398 x 8.86775
113.86775 T = 31290 + 3529.36
113.86775 T = 34819.36
T = 305.78 K .
Im stuck on these two questions anyone have the right answer?


Answers
5.) D oct-3-ol-5-en
What is a reduction reaction?
Answers
Answer:
Reduction involves a half-reaction in which a chemical species decreases its oxidation number, usually by gaining electrons. The other half of the reaction involves oxidation, in which electrons are lost. Together, reduction and oxidation form redox reactions (reduction-oxidation = redox).
Explanation:
Hope this helps :)
Math the Definitions! Please & Thank You :) Screenshot Attached.
SOMEONE HELP!
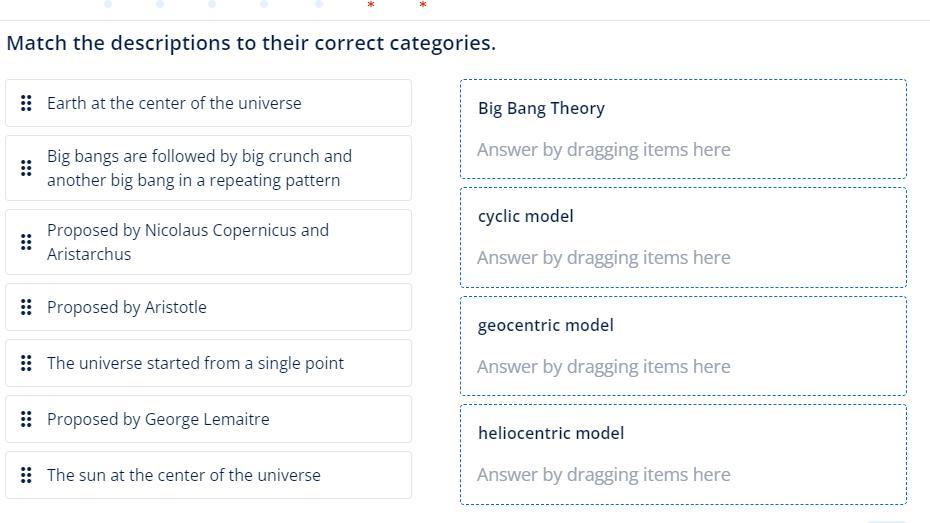
Answers
After analysing the given options and data we conclude that the matching of the given options as
1) Proposed by Nicolaus Copernicus and Aristarchus - heliocentric model
2) Proposed by Aristotle - 3) geocentric model
3) geocentric model - A) Earth at the center of the universe
4) The universe started from a single point - C) Big Bang Theory
5) Proposed by George Lemaitre - C) Big Bang Theory
6) The sun at the center of the universe - 3) geocentric model
7) heliocentric model - 6) The sun at the center of the universe
The Big Bang Theory is a scientific explanation regarding the original creation of the universe. The theory is based on several key assumptions, one of which is the isotropy of the universe. This assumption projects that the universe, more or less, appears the same in all directions of time and space.
Another key theory is that of cosmic inflation, which projects why the universe is so symmetrical on such a significant scale.
To learn more about Big bang theory
https://brainly.com/question/6841128
#SPJ1
How many moles of hydrogen are needed to completely react with 2.41 moles of nitrogen
Answers
2 good luck
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
true or false. for all atoms of the same element the 4s orbital is larger than the 3s orbital
Answers
Answer:
Between 4s and 3s orbital , 3s has more energy .
Explanation:
According to the rule , the lower the value of (n+l) for an orbital , the lower is it's energy . And if two orbitals have the same value of (n+l), the orbital with lower value of n will have the lower energy .
The statement " for all atoms of the same element the 4s orbital is larger than the 3s orbital" is definitely true.
What is an Element?An element may be defined as a type of substance that significantly cannot be dilapidated into simpler components by any non-nuclear chemical reaction. It is a type of chemical substance that bears unique properties based on numerous attributes.
The 4s orbital is the outermost and highest energy orbital, as compared to the 3s orbital. The 4s orbital penetrates more into the nucleus. This is because the s-orbital has the highest penetrating effect as compared to other orbitals.
The size of the 4s orbital is usually larger than the 3s orbital for all the atoms of the same element. This is because the electrons are filled in these orbitals systematically in the order s, p, d, and f. In the s-orbital first, 1s, 2s, 3s, 4s, and so on depending on the atomic number of the atom or element.
Therefore, the statement " for all atoms of the same element the 4s orbital is larger than the 3s orbital" is definitely true.
To learn more about S-orbitals, refer to the link:
https://brainly.com/question/24925282
#SPJ2