describe how infrared spectroscopy could be used to determine when the addition of br2 to 1-pentene had gone to completion.
Answers
Infrared spectroscopy is the analysis of infrared light interacting with a molecule. It is widely used to detect presence of functional groups and multiple bonds in organic and inorganic molecules.
There is a characterstic region of light in the absorption spectra of each compound which serves as its fingerprint. Most functional groups show absorption bands in the region (400-1400cm^-1), so it can be used to detect their presence in the molecule.
To determine whether or not the addition reaction of Bromine (Br2) with 1-pentene has gone to completion , this technique can be used as the C-H sp2 and C=C bonds spectral bands will disappear.
(To know more about IR spectroscopy : https://brainly.com/question/5951360)
Related Questions
in an equilibrium reaction with a Keq of 1×10^8,the-
A) reaction are favored
B) reaction is exothermic
C) products are favored
D) reaction is spontaneous
Answers
4) At 300. K and 1.00 atm of pressure, oxygen gas (0₂) behaves very much like an
ideal
gas. A student is working with a 3.0-L container filled with oxygen gas.
Calculate the number of moles of oxygen gas in this container. *
3 points
Answers
The number of moles of oxygen gas in the container would be 0.12 mol.
Ideal gas problemThe ideal gas equation is written as follows:
PV = nRT
Where P is the pressure of the gas, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
In this case, we are looking of n, which is the number of moles of the gas.
P is given as 1.00 atmV is given as 3.0 LT is given as 300 KR is 0.082Making n the subject of the ideal gas equation:
n = PV/RT
Substituting the values:
n = (1x3)/(0.082x300)
= 0.12 mol
In other words, the number of moles of the oxygen gas in the container is 0.12 mol.
More on ideal gas can be found here: https://brainly.com/question/3961783
#SPJ1
16. What are the two types of mixtures?
Answers
Heterogeneous and homogeneous mixtures are the two types of mixtures.
How to define the heterogeneous and homogeneous mixtures?When compared to homogeneous mixtures, heterogeneous mixtures can be visually divided into individual components. The most prevalent kind of homogeneous mixture is a solution, which can be a solid, liquid, or gas.
Solid, liquid, or gaseous homogeneous mixtures can exist. They are uniform in both appearance and chemical make-up. Water, air, steel, detergent, saltwater mixture, and other substances are examples of homogeneous mixtures. When two or more metals are combined in a specified proportion, an alloy is created.
A combination is said to be heterogeneous if its composition is not constant throughout. Vegetable soup is a complex concoction.
Know more about the mixtures at:
https://brainly.com/question/15304648
#SPJ1
Which organism is most likely to leave a fossil record?(1 point)
Responses
turtles
turtles
jellyfish
jellyfish
mushrooms
mushrooms
bacteria
FREE POINT LOLOLOL just answer correctly please TvT
Answers
Answer:
jellyfish
Explanation:
Answer: i would say jellyfish
Explanation:
just because this guy said so
(First to answer gets some good) Order the following components of the universe from largest to smallest: Moon, planet,universe,star,galaxy,nebula,comet!
Answers
Answer:
Universe, galaxy, solar system, star, planet, moon and asteroid.
Explanation:
You're welcome!
Students report that the sky in their area has mostly cirrus clouds. What are most likely the weather conditions in the area?
rainy weather
dark, overcast (blocking the sunlight)
stormy weather
fair, pleasant weather
Answers
absorptiometric determination of acid-soluble lignin in semichemical bisulfite pulp and in some woods and plants.
Answers
The absorptiometric determination of acid-soluble lignin is a method used to measure the amount of lignin in semichemical bisulfite pulp, as well as in certain woods and plants. This method involves measuring the absorption of light by the lignin in a sample.
To determine acid-soluble lignin using this method, you would first prepare a sample of the pulp, wood, or plant material. This can involve extracting the lignin using acid or other solvents. Once the sample is prepared, you would then measure its absorbance using a spectrophotometer.
The absorbance of the sample is directly proportional to the amount of lignin present. By comparing the absorbance of the sample to a standard curve, which relates known concentrations of lignin to their absorbance values, you can determine the concentration of lignin in the sample.
To know more about absorptiometry visit:
brainly.com/question/30115566
#SPJ11
Newton's First Law of Motion is...
For every force one object applies on another, there
is an equal force applied in the opposite direction.
An object at rest will stay at rest or an object in
motion will continue with the same speed and
direction if the forces acting on it are balanced

Answers
Answer:
For every force one object applies on another, there
is an equal force applied in the opposite direction.
Explanation:
please mark brainliest here
a large crystal of potassium magnet is placed in the bottom of a beaker with cold water and left for several hours
What two processes took place
Answers
Answer:
Diffusion and Dispersion.
Explanation:
The two processes that took place in the given experiment are Diffusion and Dispersion.
When a crystal of potassium magnet is added placed in the bottom of a beaker which contains cold water, after 5 minutes the particles of potassium magnet will diffuse with water through diffusion process and the colour of water turns purple.
After few hours, the colour of solution goes light purple because the tiny particles which were diffused earlier will dispersed in the water and the particles will spread and make the solution lighter in colour.
Hence, the two processes are Diffusion and Dispersion.
Choose the number of significant figures indicated 90
Answers
how does heat effect the density of an object
Answers
polease help 20pts
chemistry redox

Answers
Answer:
A is mg because it is losing 2 electrons
B is N2
C Mg as oxidation is lose of electrons
D is N2 as reduction is gain of electrons
Explanation:
you can easily remember as OIL RIG in term of electrons as Oxidation is Lost of electrons. Reduction is Gain of electrons.
which of the following is true about sodium? group of answer choices all forms of sodium are very unstable. it is most stable as an atom. it is most stable as a negatively charged ion. all forms of sodium are very stable. it is most stable as a positively charged ion
Answers
The following regarding sodium is accurate When an ion is positively charged, it is the most stable.
Ions are only the result of an atom gaining or losing electrons, is that all there is to it?In case you forgot, ions are the name for atoms that have either positive or negative charges.A negatively charged atom known as an anion is one that has gained one or even more electrons.A positively charged atom is known as a cation when one or more of its electrons have been lost.It turns into sodium quizlet when an atom loses an electron.
What differences in the kinds of compounds that atoms of the these two elements can make could explain this difference?Due to the fact that sodium has fewer electrons than protons, when it loses an electron, it changes from a neutral ion to a positively charged cation.
To know more about sodium visit:
https://brainly.com/question/29327783
#SPJ1
modern periodic table is less defective than mendeleev's periodic table. Give two reasons
Answers
Answer:
Any two reasons will be :
1:- It is based on atomic number.
2:-Problem regarding Position of isotopes is resolved.
Hope this helped
Hope this helped ALL THE BEST !!
Answer:
The modern periodic table has more info and more elements
Explanation:
3)What helps the plants to receive sunlight in tropical rainforests?
Answers
Answer:
Large leaves help plants to receive more sunlight when in tropical rainforests.
water is a(n) ___________ coolant for machinery and engines because of its ___________ specific heat.
Answers
Water is a good coolant for machinery and engines because of its high specific heat.
What is specific heat?The specific heat of a substance is the amount of energy required to raise the temperature of one gram of the substance by one degree Celsius or Kelvin. The specific heat of water is 4.184 J/g°C, which is relatively high compared to other liquids.
As a result, water is an excellent coolant for machinery and engines because it can absorb a large amount of heat without significantly raising its own temperature.
Hence, water is a good coolant for machinery and engines because of its high specific heat.
Read more about Machinery at https://brainly.com/question/9806515
#SPJ11
Help please now ASAP please need help noww

Answers
A chemical equation is a symbolic representation of a chemical reaction where reactants are represented on the left, and products on the right.
A chemical equation is said to be balanced when the number of atoms of each element on both sides of the equation are the same.
According to this question, chlorine gas reacts with pottasium iodide to produce iodine and pottasium chloride as follows:
Cl₂ + 2KI → 2KCl + I₂
Therefore, the answers to the questions are as indicated in the main answer part.
Learn more about balanced equation at: https://brainly.com/question/28294176
#SPJ1
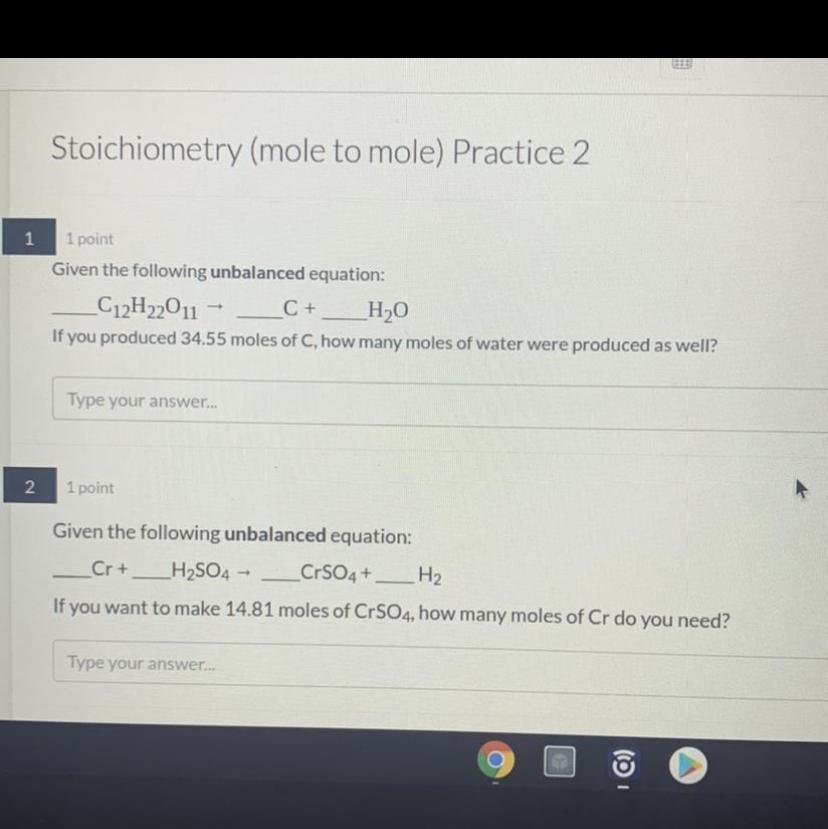
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
1. Given the unbalanced equation:
C12H22O11 = C + H2O
If you produced 34.55 moles of C, how many moles of water were produced as well?
2. Given the unbalanced equation:
Cr + H2SO4 = CrSO4 + H2
If you want to make 14.81 moles of CrSO4, how many moles of Cr do you need?
Answers
Answer:
1. 31.68 moles of water, H₂O
2. 14.81 moles of Cr
Explanation:
1. Determination of the number of mole of water, H₂O.
The balanced equation for the reaction is given below:
C₁₂H₂₂O₁₁ —> 12C + 11H₂O
From the balanced equation above,
1 mole of C₁₂H₂₂O₁₁ produced 12 moles of C and 11 moles of H₂O.
Next, we shall determine the number of mole C₁₂H₂₂O₁₁ needed to produce 34.55 moles of C. This can be obtained as follow:
From the balanced equation above,
1 mole of C₁₂H₂₂O₁₁ produced 12 moles of C.
Therefore, Xmol of C₁₂H₂₂O₁₁ will produce 34.55 moles of C i.e
Xmol of C₁₂H₂₂O₁₁ = 34.55 / 12
Xmol of C₁₂H₂₂O₁₁ = 2.88 moles
Thus, 2.88 moles of C₁₂H₂₂O₁₁ is needed.
Finally, we shall determine the number of mole of water, H₂O produced from the reaction. This can be obtained as follow:
From the balanced equation above,
1 mole of C₁₂H₂₂O₁₁ produced 11 moles of H₂O.
Therefore, 2.88 moles of C₁₂H₂₂O₁₁ will produce = 2.88 × 11 = 31.68 moles of H₂O.
Thus, 31.68 moles of water, H₂O were obtained from the reaction.
2. Determination of the number of mole of Cr needed.
The balanced equation for the reaction is given below:
Cr + H₂SO₄ —> CrSO₄ + H₂
From the balanced equation above,
1 mole of Cr reacted to produce 1 mole of CrSO₄.
Finally, we shall determine the number of mole of Cr needed to produce 14.81 moles of CrSO₄. This can be obtained as follow:
From the balanced equation above,
1 mole of Cr reacted to produce 1 mole of CrSO₄.
Therefore, 14.81 moles of Cr will also react to produce 14.81 moles of CrSO₄.
Thus, 14.81 moles of Cr is needed for the reaction.
What will be the final temperature of a 10.0 g piece of iron ( c = 0.450 J g -1 °C -1) initially at 25°C, if it is supplied with 9.5 J from a stove?
Answers
The final temperature of a 10.0 g piece of iron initially at 25°C, if supplied with 9.5 J from a stove, is 27.11°C. The change in temperature of the iron is 2.11°C.
We can use the formula:
Q = mcΔT
where Q is the heat absorbed by the iron, m is the mass of the iron, c is the specific heat capacity of iron, and ΔT is the change in temperature.
In this case, the heat absorbed by the iron is 9.5 J, the mass of the iron is 10.0 g, the specific heat capacity of iron is 0.450 J g^(-1) °C^(-1), and the initial temperature is 25°C. We want to find the final temperature.
Let's rearrange the formula to solve for ΔT:
ΔT = Q / mc
Substituting the given values, we get:
ΔT = (9.5 J) / (10.0 g x 0.450 J g^(-1) °C^(-1))
ΔT = 2.11 °C
Therefore, the change in temperature of the iron is 2.11°C.
To find the final temperature, we add the change in temperature to the initial temperature:
T_final = T_initial + ΔT
T_final = 25°C + 2.11°C
T_final = 27.11°C
Therefore, the final temperature of the iron is 27.11°C.
Learn more about iron: https://brainly.com/question/18500540
#SPJ11
give all possible values of l for the second principal level (n = 2).
Answers
Answer: In the Bohr model of the atom, the principal quantum number (n) describes the energy level of the electron, and the azimuthal quantum number (l) describes the angular momentum of the electron. The allowed values of l for a given value of n are integers ranging from 0 to (n-1). Therefore, for the second principal level (n = 2), the possible values of l are:
l = 0, 1
So the allowed values of l for the second principal level are 0 and 1. Note that the value of l also determines the shape of the orbital, with l = 0 corresponding to an s orbital and l = 1 corresponding to a p orbital.
Explanation: In the Bohr model of the atom, the principal quantum number (n) describes the energy level of the electron, and the azimuthal quantum number (l) describes the angular momentum of the electron. The allowed values of l for a given value of n are integers ranging from 0 to (n-1). Therefore, for the second principal level (n = 2), the possible values of l are:
l = 0, 1
So the allowed values of l for the second principal level are 0 and 1. Note that the value of l also determines the shape of the orbital, with l = 0 corresponding to an s orbital and l = 1 corresponding to a p orbital.
Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters
Answers
The correct order of the increasing polarity of the analyte functional group isEthers < Esters.
The given statement is "Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters." The order of polarities of functional groups is the order of their increasing polarity (i.e., less polar to more polar) based on their electron-donating or withdrawing ability from the rest of the molecule.Polarity of analyte: The analyte's polarity is directly proportional to the dipole moment of the functional group, which is associated with a difference in electronegativity between the atoms that make up the functional group.The electronegativity of an element is its ability to attract electrons towards itself. The greater the difference in electronegativity between two atoms, the more polar their bond, and hence the greater the polarity of the molecule.
To find the correct order of the increasing polarity of the analyte functional group, let's first compare the two groups: hydrocarbon ethers and esters. Here, esters have a carbonyl group while ethers have an oxygen atom with two alkyl or aryl groups. The carbonyl group has more electronegative oxygen, which pulls electrons away from the carbon atom, resulting in a polar molecule. On the other hand, ethers have a less polar oxygen atom with two alkyl or aryl groups, making them less polar than esters. Therefore, the correct order of the increasing polarity of the analyte functional group isEthers < Esters.
To know more about polarity visit:-
https://brainly.com/question/33242453
#SPJ11
The general formula for ____________ and ____________ are cnh2n 2 and cnh2n , respectively.
Answers
The general formula for alkenes and alkynes are cnH2n and cnH2n-2, respectively.
Alkenes are hydrocarbons with a double bond made of carbon and carbon (C=C). Alkenes have the generic formula cnH2n, where "n" stands for the molecule's number of carbon atoms.
In an alkene, each carbon atom is joined to two hydrogen atoms, two other carbon atoms, and one oxygen atom.
For instance, ethene (commonly known as ethylene), which contains two carbon atoms, has the general formula C2H4.
Alkynes are hydrocarbons with a triple bond made of carbon and carbon. Alkynes have the generic formula cnH2n-2. Similar to alkenes, the letter "n" designates how many carbon atoms are present in the molecule. In an alkyne, each carbon atom is joined to one hydrogen atom and one additional carbon atom.
For instance, the usual formula for ethyne, which also goes by the name acetylene and includes two carbon atoms, is C2H2.
know more about hydrocarbons here
https://brainly.com/question/30666184#
#SPJ11
Instructions:
Balance chemical equations on bach side by multiplying the molecular or ionic compounds by a
coefficient.
_C3_H8
+
O2
CO2
H2O
Answers
Answer: the answer is b
Explanation:
Answer:
Explanation:
B answer
Which are the physical properties of water
Answers
Answer:
Some physical properties of water:
It is odorless.It could appear as a white crystalline solid in solid form, a transparent gas with almost no color but a slight hint of blue in liquid form, or a colorless gas in gas form.It has a melting point of 0°C and a boiling point of 100°C.Its density (at 25°C) is about 0.99701 grams/cm³.It has a viscosity of 0.8903 centi-poise.Of course, there are many more but these are some common ones.
What is a concern of the survey method of research?
1.the target population will not be able to be identified
2.the number of people in the survey may be too large
3.the researcher is dependent on the person answering the survey to be honest
4.all answers are correct
Answers
Concern of the survey method of research is : 3.)the researcher is dependent on the person answering the survey to be honest.
What is a concern of the survey method of research?One concern of the survey method of research is that the researcher is dependent on the person answering the survey to be honest. There is a risk that participants may provide inaccurate or incomplete responses due to range of factors such as social desirability bias, memory recall issues, or misunderstanding of questions.
Therefore, option 3)the researcher is dependent on the person answering the survey to be honest is the most accurate answer.
To know more about survey method, refer
https://brainly.com/question/13435620
#SPJ1
how can you tell the charge of an ion?
Answers
Answer:
the charge of an element is equal to the number of orotons minus the number of electrons. the number if proton is equal to the number of elements given in the periodic table. the number of electrons is equal to the atomic numver minus the CHARGE of the atom
Q1 and Q1 represent ?
Answers
Answer:yes they do represent
Write a nuclear equation for the beta decay of the following isotopes: Lead-210
Answers
In this question, we need to determine what will be the nuclear equation for the Beta decay of Lead - 210.
Beta decay is a type of radioactive decay in which we will have either a neutron becoming a proton, this will be called Beta-minus decay or a proton becoming a neutron, this will be called a Beta-plus decay
In the case of Pb-210, we will have a Beta-minus decay, and one neutron will become a proton:
210/82 Pb ---> 210/83 Bi + electron
Lead - 210 will become Bismuth - 210
If you could change one thing about distance learning, what would it be?
Answers
Answer:
Nothing
Explanation:
BECAUSE DONT ASK
What forces typically hold ions together?
O A. Intermolecular forces
OB. Ionic attractions
OC. Metallic bonds
O D. Covalent bonds
Answers
Answer: Ionic attractions
Explanation:
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.