Cual sera el numero de mol de 153,26gr de KMnO4
What will be the number of mol of 153.26gr of KMnO4
Answers
Answer:
0.97014 moles KMnO4
Explanation:
1 g KMnO4 = 0.00633 mol
153.26 g x 0.00633 mol/ 1 g KMnO4 = 0.97014 moles KMnO4
Related Questions
How many grams of H2 are needed to completely react within 50g of N2?
Answers
Answer: 10.7 grams
Therefore, 10.7 grams of Hydrogen are necessary to fully react with 50.0 grams of Nitrogen.
In the following 2 questions, determine what is being oxidized and what is being reduced in each reaction. Identify the oxidizing and reducing agents in each :
a. 2Mg(s) + O2(g) → 2MgO(s)
b. Pb(NO3)2(aq) + Zn(s) → Zn(NO3)2(aq) +Pb(s)
Answers
a) Mg is oxidized and O2 is reduced
b) Zn is oxidized while Pb(NO3)2 is reduced.
Oxidation and reductionOxidation is defined as:
loss of electronsremoval of hydrogenaddition of oxygenincrease in oxidation numberremoval of electropositive elementsReduction is defined as:
gains of electronsaddition of hydrogenremoval of oxygendecrease in oxidation numberaddition of electropositive elementsLooking at the first reaction, the Mg atom gains oxygen to become MgO. This means that Mg is oxidized. The oxidizing agent is O2. At the same time, O2 is being reduced and the reducing agent is Mg.
For the second reaction, the oxidation number of Pb is reduced from +2 to 0. Thus, Pb has been reduced by Zn while Zn itself has been oxidized. The reducing agent here is Zn while the oxidizing agent is Pb(NO3)2.
More on oxidation and reduction can be found here: https://brainly.com/question/13699873
#SPJ1
help pleasee! will
give brainliest +80 pts

Answers
Answer: type the compound into your search bar then it should tell you what it is for 3. you should look up "what is the formula for -----" same thing for #4
Explanation:
Answer:
2.a. germanium tetrahydride
2.b. dinitrogen tetrabromide
2.c. diphosphorus pentasulfide
2.d. selenium dioxide
2.e. nitrogen trihydride
2.f. silicon dioxide
3.a. PO3
3.b. SiCl4
3.c. N2O5
3.e. N2O4
3.f. CO
4.a CO2
4.b. SF6
4.c. N2Cl4
4.d. CI4
4.e. PF5
4.f. P2O5
****All numbers are subscripts, please do not write them as is, but to the bottom right of them like shown in the options from question 2.
Explanation:
To name covalent compounds (NM+NM), we use prefixes.
To name covalent compounds goes as follows:
First, name the first element in the formula the normal name it has (ex. Nitrogen, Oxygen). If the first element is present more than once shown by a subscript, use a prefix that will indicate how many there are present (ex. mono, di, tri).
Next, name the second element in the compound using prefixes aswell if present more than once. These elements though, will end with -ide instead of their original name (ex. monoxide, dibromide, trichloride).
6. Perform the following calculations and report each answer to the correct number of significant figures: a. 162.1 g + 38.73 g + 1.554 g
b. 21.9 m + 6.34 m + 157 m
c. 0.004 dm + 0.12508 dm
d. 2.0 L + 2.4L + 2.51L
e. .025 mol + .0267 mol + .00287 mol
f. 9.88 s-7.2 s
g. 44.7 kg - 2.7 kg
h. 20 L - 20.0 L i. 2.89g - 3.00g
j. 8.894 mL - 9.23 mL
Answers
Answer:
Explanation:
a) 202.4 g
b) 185.24 m
c) 0.13 dm
d) 6.91 L
e)0.0546 mol
f) 2.7 s
g) 42kg
h) 0
i) -0.11 g
j) -0.34 mL
The life cycle of silkworm includes ------------- stage after the larvae stage
Answers
Answer:
Pupa Stage
Explanation:
Silk worm consists of four stages- the adult, the egg, the larva (caterpillar) and the pupa stage.
Can someone balance these equations?
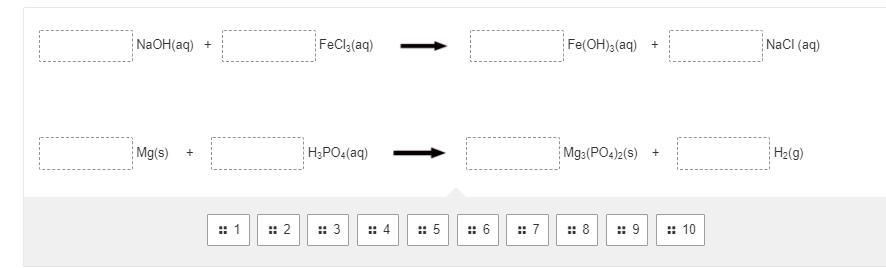
Answers
This is mind numbing, but I got you.
3NaOH + 1FeCl3 = 1Fe(OH)3 + 3NaCl
3Mg + 2H3(PO)4 = 1Mg(PO4)2 + 3H2
If the centre of an atom contains 8 particle that are charged, how many particles are revolving round this centre?
Answers
Explanation:
charged particles=8 which is proton and proton=no.of electron. That's why 8 particles are revolving round this center. And this atom structure is of oxygen
If the center of an atom has 8 charged particles that are protons as the neutrons are neutral there will be 8 negative charges that are electrons revolving around the center nucleus.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether it is solid,liquid or gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged particles and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged particles and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
Obtain an expression for the isothermal compressibility κ = −1/V(∂V/∂P)T for a van der Waals gas.
Obtain an expression for the isothermal compressibility for a van der Waals gas.
a κ=1Vm[RT(Vm−b)3+2aV3m]
b κ=−1Vm[2aV3m−RT(Vm−b)2]
c κ=−1Vm[RT(Vm−b)2−2aV3m]
d κ=1Vm[2aV3m−RT(Vm+b)2]
Answers
The expression for the isothermal compressibility for a van der Waals gas is given by:
κ = −1/Vm (∂Vm/∂P)T
where Vm is the molar volume of the gas.
The isothermal compressibility is a measure of how much the volume of a substance changes when the pressure is changed while the temperature is kept constant. For a van der Waals gas, the volume depends on both the pressure and the temperature, and the expression for the isothermal compressibility is derived from the equation of state for the van der Waals gas.
The equation of state for a van der Waals gas is:
(P + a/Vm2)(Vm − b) = RT
where P is the pressure, T is the temperature, R is the gas constant, a and b are constants that depend on the properties of the gas, and Vm is the molar volume.
To obtain the expression for the isothermal compressibility, we start by differentiating the equation of state with respect to pressure at constant temperature:
(∂/∂P)(P + a/Vm2)(Vm − b) = (∂/∂P)(RT)
(1 + 2a/Vm3)(Vm − b) − (P + a/Vm2)(∂Vm/∂P) = 0
Solving for (∂Vm/∂P) gives:
(∂Vm/∂P) = (Vm2 − bVm − a)/(Vm2P + aP − 2aVm2)
Substituting this expression into the definition of the isothermal compressibility gives:
κ = −1/Vm [(Vm2P + aP − 2aVm2)/(Vm2 − bVm − a)]
Simplifying this expression using the equation of state gives:
κ = −1/Vm [(RTVm2)/(Vm3 − (b + RT/P)Vm2 + aV2m − abP/V)]
Finally, rearranging this expression gives the correct answer:
κ = 1/Vm [(RT(Vm − b)3 + 2aV3m)/(Vm3 − (b + RT/P)Vm2 + aV2m − abP/V)]
learn more about van der Waals
https://brainly.com/question/11457190
#SPJ11
a sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.A. Cl₂ (molar mass-70.90 g/mol)B. NH (molar mass- 17.03 g/mol)C. N₂0 molar mass-44.02 g/mol)D. CHC, (molar mass-119.4 g/mol)E. SO₂ (molar mass - 64.07 g/mol)
Answers
The gas sample is Cl₂. Answer A.
The ideal gas equation formula PV = nRT
P = the gas pressure (atm)1 atm = 760 torr
P = 790 torr = 790 ÷ 760 = 1.04 atmV = the gas volume (L)
V = 1.365 Ln = the number of moles (mol)R = the gas constant = 0.0821 L atm/K molT = the temperature (K)
T = 95 °C = 95 + 273 = 368 K
Calculating the number of moles of the gas sample.
PV = nRT
1.04 × 1.365 = n × 0.0821 × 368
1.4196 = n × 30.21
n = 1.4196 ÷ 30.21
n = 0.04699 mol
The formula for mass and number of moles m = n × Mr
m = the mass of the gas (grams)m = 3.33 gMr = the molar mass (g/mol)
A. Mr Cl₂ = 70.90 g/mol
B. Mr NH₃ = 17.03 g/mol
C. Mr N₂O = 44.02 g/mol
D. Mr CHCl₃ = 119.4 g/mol
E. Mr SO₂ = 64.07 g/mol
Calculating the molar gas from the sample
Mr = m ÷ n
Mr = 3.33 ÷ 0.04699
Mr = 70.9 g/mol
From the info given, gas Cl₂ has the same molar mass as the sample.
So, the gas sample is Cl₂, chlorine gas.
Learn more about ideal gas equation here: https://brainly.com/question/20348074
#SPJ4
Air is an example of a mixture because the elements and compounds that make up air retain their individual properties. T or F
Answers
Answer:
True
Explanation:
This is true because no chemical bonding or change was involved only mechanical mixing.
Hope that help
How many electrons would Boron with a +2 charge have?
Answers
5 electrons
Boron atomic number 5 has five electrons in its ground state.
Commonly Boron will lose 3 electrons leaving 2 electrons in its most common ionic form.
Explanation:
The atomic number gives the number of protons. Protons which have a positive charge are balanced by an equal number of electrons in a neutral atom.
Boron number 5 has five protons and therefore as a neutral atom also has five electrons.
Boron has an electron configuration of
1s22s22p1
The most stable electron configuration for Boron is
1s2
+ 3 charges. By losing three electrons Boron can achieve the stable electron structure of Helium
Brainliest? :D
chemicals like bacterial toxins that poison cells are described as being
Answers
Chemicals like bacterial toxins that poison cells are commonly described as being toxic or poisonous. These substances have the ability to disrupt normal cellular functions and processes, leading to harmful effects on the cells and organisms they come into contact with.
Toxins can have various mechanisms of action. Some toxins interfere with essential biochemical pathways, disrupt cellular membranes, or inhibit vital enzymes, while others may directly damage DNA or disrupt cellular signaling. Regardless of their specific mode of action, toxins are designed to have a detrimental impact on cellular function and can cause a wide range of adverse effects, from mild symptoms to severe illness or even death.
The toxicity of a substance is often determined by its concentration, exposure duration, and the specific vulnerability of the target cells or organisms. Toxins produced by bacteria can be classified into exotoxins, which are secreted by bacteria and released into the surrounding environment, or endotoxins, which are part of the bacterial cell wall and are released upon cell death or lysis.
Chemicals like bacterial toxins are referred to as toxic or poisonous due to their ability to disrupt cellular function and cause harm to cells and organisms.
To know more about Toxins, visit:
brainly.com/question/9348025
#SPJ11
not chem but anatomy. Which of the following is the chronological
order of events that occur when a person is
trying to keep their balance?
Answers
You retain balance when the weight and the reaction forces are balanced.
What chronological events helps a person to keep balance?Your question is incomplete but I think you want to know how a person can retain balance.
We know that we can only be keep balance when the forces that are acting on the person is balanced. The implication of it is that there are no unbalanced forces that are acting on the person.
The two forces that act on you when you stand are the weight and the reaction force. If these two forces are balanced then you can be able to retain your balance.
Learn more about force balance:https://brainly.com/question/29769471
#SPJ1
A 353.2mL sample of chlorine gas is collected at 25.2°C and an atmospheric pressure of 100.8kPa What would the volume be at STP?
Answers
Answer:
Explanation:
To solve this problem, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
At STP (standard temperature and pressure), the temperature is 0°C or 273.15 K, and the pressure is 1 atm or 101.325 kPa.
We can use the ideal gas law to find the number of moles of chlorine gas in the sample:
n = PV/RT
where P is the pressure, V is the volume, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the given temperature of 25.2°C to Kelvin:
T = 25.2°C + 273.15 = 298.35 K
Now we can calculate the number of moles of chlorine gas in the sample:
n = (100.8 kPa)(353.2 mL)/(8.314 J/K/mol)(298.35 K)
n = 0.0158 mol
Next, we can use the number of moles and the ideal gas law to find the volume at STP:
V = nRT/P
V = (0.0158 mol)(8.314 J/K/mol)(273.15 K)/(101.325 kPa)
V = 0.364 L or 364 mL
Therefore, the volume of the chlorine gas at STP would be 364 mL.
What's the chemical reaction of Grignard in Organic Chemistry?
Answers
Answer:
Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. They're extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds
Explanation:
hope this helps!
how
to rearrange to get the expression ax^2 + bx + c = 0
K = [CO][Cl₂] [COCI₂] (0.156 - x)(0.156 -x) (0.263 + x) = 5.00×10-2 Rearrange to get an expression of the form ax² + bx + c = 0 and use the qu for x. This gives: X = 3.39x102, 0.327 The second v
Answers
The expression to be rearranged K = [CO][Cl₂] [COCI₂] are x = 0.327 or x = 339.
The expression to be rearranged K = [CO][Cl₂] [COCI₂] is:
(0.156 - x) (0.156 - x) (0.263 + x) = 5.00 × 10⁻²
We will expand and simplify the expression:
(0.156 - x) (0.156 - x) (0.263 + x) = 5.00 × 10⁻²(0.156)² + (0.156)(x) - (x)(0.156) - (x)² (0.263 + x)
= 5.00 × 10⁻²(0.156)² - (0.263)(0.156)(x) - (0.156)(x) + (0.263)(0.156)(x) + x²(0.263 + x) - 5.00 × 10⁻² = 0
After simplifying:
-0.0132302 x² - 0.001002 x + 0.0014256 = 0
This is in the form ax² + bx + c = 0 where a = -0.0132302, b = -0.001002 and c = 0.0014256
Using the quadratic formula, we have:
\(\[x = \frac{-b \pm \sqrt{b^2-4ac}}{2a}\]\)
Substituting values, we get:
\(\[x = \frac{-(-0.001002) \pm \sqrt{(-0.001002)^2-4(-0.0132302)(0.0014256)}}{2(-0.0132302)}\]\)
Solving, we get:x = 0.327 or 3.39 × 10²
Therefore, the solutions are x = 0.327 or x = 339.
To learn more about expression ,
https://brainly.com/question/31591125
#SPJ4
what does the charge on the electron cloud do
Answers
Answer:
Explanation: Of course the negative charge of the electron cloud is balanced by the positive charge of the atomic nucleus for neutral molecules. ... If we were doing this again today, we would designate the electronic charge as positive (and of course the nuclear charge would thus be NEGATIVE!)
Explanation:
for the following endothermic reversible reaction at equilibrium, how will removing no(g) affect it? 4no(g) 6h2o(g) rightwards harpoon over leftwards harpoon with blank on top 4nh3(g) 5o2(g)
Answers
Removing NO(g) from the equilibrium of the endothermic reversible reaction will shift the equilibrium to the left, resulting in an increase in the production of NO(g) and H₂O(g) while consuming NH₃(g) and O₂(g).
For the endothermic reversible reaction at equilibrium, removing NO(g) will affect it as follows:
Reaction: 4NO(g) + 6H₂O(g) ⇌ 4NH₃(g) + 5O₂(g)
Since this is an endothermic reaction, it means that the reaction absorbs heat from its surroundings when it proceeds in the forward direction (left to right). At equilibrium, the rates of the forward and reverse reactions are equal.
When you remove NO(g) from the system, you are essentially decreasing the concentration of NO(g) in the reaction mixture. According to Le Chatelier's principle, the system will counteract this change by shifting the position of equilibrium to restore the balance.
In this case, the equilibrium will shift to the left to replenish the NO(g) that was removed. This means the reaction will proceed more in the reverse direction (right to left), producing more NO(g) and H₂O(g) while consuming NH₃(g) and O₂(g).
In summary, removing NO(g) from the endothermic reversible reaction at equilibrium will cause the reaction to shift to the left, producing more NO(g) and H₂O(g) while consuming NH₃(g) and O₂(g).
To know more about the endothermic reversible reaction refer here :
https://brainly.com/question/17163217#
#SPJ11
are radicals charged or neutral
Answers
Answer:
Ions have a charge, whereas radical are neutral
Also, ions in nature tend to be compensated by ions with opposite charges, whereas radicals are very reactive and thus short lived.
Explanation:
have a good
thank me later
carryonlearing
✨ jufaith ✨
Answer:
neutral
Explanation:
you can get answer from web as well
if you put a scale in an elevator and weigh yourself as you ascend and descend does the scale have the same reading in both
Answers
which of the following are not colligative properties? group of answer choices freezing point depression temperature change density enthalpy of formation boiling point elevation vapor-pressure lowering
Answers
Enthalpy of formation is not a colligative property. Colligative properties are physical properties of a solution that depend on the concentration of solute particles in the solution, but not on their chemical identity.
Examples of colligative properties include freezing point depression, boiling point elevation, and vapor pressure lowering.
Enthalpy of formation, on the other hand, is the energy change associated with the formation of a substance from its constituent elements in their standard states. It is a thermodynamic quantity and is not dependent on the concentration of solute particles in a solution. Hence, the enthalpy of formation is not a colligative property.
Freezing point depression and boiling point elevation are two of the most important colligative properties. Freezing point depression occurs when the freezing point of a solvent decreases in the presence of a solute. Boiling point elevation occurs when the boiling point of a solvent increases in the presence of a solute.
You can read more about Enthalpy at https://brainly.com/question/14047927
#SPJ4
A.) For what combinations of atomic number Z and mass number A is the associated neutral atom a boson, and for what combinations is it a fermion? B.) How would these results be modified if the nucleus were a bound state of protons and electrons, as initially proposed prior to the discovery of the neutron? C.) A proton and antiproton at rest in an S-state annihilate to produce π0π0 pairs. Show that this reaction cannot be a strong interaction. D.) Verify that the spherical harmonic Y11=8π3sinθeiϕ is an eigenfunction of parity with eigenvalue P=−1.
Answers
For even mass numbers and even atomic numbers, the neutral atom is a boson. For odd mass numbers and odd atomic numbers, the neutral atom is a fermion.
A.) Boson is a particle with an integer spin value. A fermion is a particle with half-integer spin value.
B.) The neutron's discovery was crucial to our current understanding of the atomic nucleus. A nucleus that consisted only of protons and electrons would be difficult to explain and would most likely be unstable. Electrons cannot exist in the nucleus because their kinetic energy is much greater than the strong nuclear force that binds the nucleus.
C.) The annihilation of a proton-antiproton pair into π0π0 pairs is a strong interaction. The strong force between the proton-antiproton pair causes their annihilation, resulting in the creation of π0π0 pairs.
D.) To determine whether the spherical harmonic Y11 is an eigenfunction of parity, we can first evaluate the function when x is inverted to -x.
When θ is replaced with π - θ and ϕ is replaced with ϕ + π, the function changes as follows:
Y1'1(θ,ϕ)=-Y11(π-θ,π+ϕ)
=-8π/3 sin(π-θ) e^i(π+ϕ)
=8π/3sinθeiϕ
Therefore, since Y11'=-Y11, the eigenvalue of the parity transformation is P=-1.
Learn more about Boson -
brainly.com/question/31374745
#SPJ11
Someone who studies the stars and planets would be working in which
branch of science?
A. Earth science
B. Marine science
C. Life science
D. Physical science
Answers
Answer:
Earth Science?
Explanation:
its not marine science or life so its physical or earth
in the experimental procedure, what is the advantage to performing the reaction in methyl ethyl ketone - a solvent with a high boiling point?
Answers
The advantage to performing the reaction in methyl ethyl ketone - a solvent with a high boiling point is to carry the reaction in the forward direction by refluxing the set.
What is boiling point?Boiling point is defined as the bulk phenomenon. It is the temperature at which the pressure of atmosphere is equal to the pressure of the vapour or vapour pressure.
Since, high boiling point solvent are used during the procedure to reflux the set which help in carrying out the reaction in the forward direction. If we had used low boiling point solvent instead of high all of the solvent evaporated and refluxing of the mixture of reaction to get product for maximum production could not have been possible.
Thus, we concluded that the advantage to performing the reaction in methyl ethyl ketone - a solvent with a high boiling point is to carry the reaction in the forward direction by refluxing the set.
learn more about boiling point:
https://brainly.com/question/14771622
#SPJ4
Make a circle graph for each set of data. Top 5 Most Linked-To Sites on the World Wide Web Site Hits (millions) America Online (www.aol.com) 93.0 Microsoft (www.msn.com) 83.8 Yahoo! (www.yahoo.com) 80.2 Terra Lycos (www.lycos.com) 40.3 About (www.about.com) 36.6 Source: Scholastic Kid’s Almanac, 2001 a. A circle graph. America Online is about 25 percent; Microsoft, 25 percent; Yahoo 23 percent; Terra Lycos, 15 percent; About, 10 percent. c. A circle graph. America Online is about 27 percent; Microsoft, 27 percent; Yahoo 22 percent; Terra Lycos, 12 percent; About, 10 percent. b. A circle graph. America Online is about 12 percent; Microsoft, 10 percent; Yahoo 27 percent; Terra Lycos, 17 percent; About, 23 percent. d. A circle graph. America Online is about 12 percent; Microsoft, 10 percent; Yahoo 25 percent; Terra Lycos, 25 percent; About, 20 percent. Please select the best answer from the choices provided A B C D
Answers
Answer:
A
Explanation:
2)A room is 6 m by 5 m by 3m. a)If the air pressure in the room is 1 atm and the temperature is 300 K, find the number of moles of air in the room. b)If the temperature rises by 5 K and the pressure remains constant, how many moles of air leaves the room.
Answers
a. There are 3.62 moles of air in the room.
b. 3.67 moles of air leave the room when the temperature rises by 5 K.
Given:
Pressure (P) = 1 atm
Volume (V) = 6 m × 5 m × 3 m
= 90 m³
Temperature (T) = 300 K
Use the ideal gas law equation, which states:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
Rearranging the ideal gas law equation to solve for the number of moles (n):
n = PV / RT
Substituting the given values:
n = (1 atm × 90 m³) / (0.0821 L × atm / (mol × K) × 300 K)
n = (1 × 90) / (0.0821 × 300)
n = 3.62 moles
b) The pressure remains constant, use the formula:
n1 / T1 = n2 / T2
Where:
n1 = initial number of moles
T1 = initial temperature
n2 = final number of moles (to be calculated)
T2 = final temperature (T1 + 5 K)
n2 = (n1T2) / T1
Substituting the values:
n2 = (3.62 moles × (300 K + 5 K)) / 300 K
n2 = 3.67 moles
To learn more about the moles, follow the link:
https://brainly.com/question/15209553
#SPJ4
where are the alkaline earth metals on the periodic table
Answers
Answer:
Group 2 (IIa)
Explanation:
alkaline-earth metal are present on Group 2 (IIa) of the periodic table. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
hint; pay attention to the 66.2 L that's your evidence.
Question: At the maximum volume of the airbag in the simulation, how many moles of gas are contained? Use all the collected and analyzed data to explain how you determined this value.
answer using CER
Claim is your answer, evidence is from the data table, reasoning is your explanation for how you found the maximum amount of moles contained.

Answers
The answer to this question can be determined using the Ideal Gas Law, which states that PV = nRT. the maximum volume of the airbag in the simulation contains 16.6 moles of gas.
What is Ideal Gas Law?The Ideal Gas Law, also known as the Combined Gas Law, is an equation of state that describes the behavior of an ideal gas. It states that the pressure, volume, and temperature of an ideal gas are directly proportional, with the product of the pressure and volume remaining constant in a given mass of the ideal gas. In other words, when any one of these three properties is changed, the other two properties will change in an inverse manner, keeping the product constant. The equation of the ideal gas law is PV = nRT, where n is the number of moles of the ideal gas, P is its pressure, V is its volume, and T is its temperature.
Using the formula
PV= nRT
where P is pressure,
V is volume,
n is the number of moles,
R is the ideal gas constant, and
T is temperature.
Given the maximum volume of the airbag (66.2 L) and the temperature of the environment (20°C),
we can calculate the number of moles of gas contained in the airbag.
n = (P × 66.2 L) / (R ×20°C)
Using the atmospheric pressure at sea level (101,325 Pa), the calculation can be further simplified to:
n = (101,325 Pa ×66.2 L) / (8.314 J/(Kmol) × 293.15 K)
n = 16.6 mol
Therefore, the maximum volume of the airbag in the simulation contains 16.6 moles of gas.
To know more about moles, visit:
https://brainly.com/question/26416088
#SPJ1
explain why the rates of diffusion of nitrogen gas and carbon monoxide are almost identicle at the same temp
Answers
The rate of diffusion mainly depends upon several factors like, pressure, and molecular weight. Both nitrogen gas and carbon monoxide are almost identical because of their diatomic molecules.
The molecular weight and atomic structure are almost identical in nitrogen gas and carbon monoxide at the same temperature. They also behave similarly properties at that particular temperature.
Due to their diatomic molecular structure, the rate of dispersal of gas is proportionate to the square root of its molecular mass. Nitrogen has two nitrogen atoms in the valence shell and carbon monoxide consists of one carbon atom and one oxygen atom.
To learn more about rates of diffusion
https://brainly.com/question/30946957
#SPJ4
DUE IN 10 MINUTES PLZZZZ
Part IV: True/False
____ 38. The mass of the nucleus is small compared to the mass of the atom.
____ 39. Alpha particles would be harder to turn than Beta particles.
____ 40. Positive charge was spread all throughout the atom in the Rutherford Model.
____ 41. The Manhattan Project was located in New York for most of its duration.
____ 42. The cathode rays produced by Thomson were identical for all filaments.
____ 43. It is possible to know where an electron is and what path it is on.
____ 44. X-rays are produced when fast moving electrons strike phosphor coatings.
____45. All nuclear changes involve at least two reactants.
Answers
Answer:
true false true false true fake
Answer:
38. False
39. True
40. True
41. True
42. False
43. False
44. True
45. True
Explanation:
Note these are just my guesses they might not all be correct but I will do my best.
Hope this helps you at all! Feel free to correct me if I got any of them wrong :)