cosi2 is formed by a cobalt thin-film reaction with silicon. what is the position of the cosi2 surface relative to the original silicon surface?
Answers
the cosi2 surface is in contact with the original silicon surface, which means the original silicon surface is under the cosi2 surface.
Cosi2 is an intermetallic compound formed from the reaction of cobalt (Co) and silicon (Si). Cobalt silicide, also known as cosi2, is a compound of cobalt and silicon that is frequently used in the semiconductor industry. It is created by the reaction of cobalt with silicon, forming a thin film.
It is known to be one of the most extensively used contact materials, with a low contact resistance and good thermal stability.Silicon and cobalt are initially layered on top of one another to form a cosi2 layer. This can be done in a few different ways, including sputtering or evaporation. In general, the silicon is deposited on top of the cobalt. The reaction between the two metals, which results in the formation of cosi2, then occurs. As a result, the cosi2 layer is in contact with the original silicon surface, and the silicon surface is under the cosi2 layer.
TO know more about that silicon visit:
https://brainly.com/question/14505564
#SPJ11
Related Questions
Why does the developing chamber need to be prepared before adding the tlc plate?.
Answers
The developing chamber needs to be prepared because the solutions of the solute and solvent to dissolved.
What is a TLC plate?
TLC Plate Buying Guide Thin-layer chromatography (TLC), a standard analytical method for separating and identifying the chemicals in a given mixture, can also be used to assess the purity of a specific component included within that mixture. Gypsum and 60 G TLC Silica Gel Plates
What is the chamber process?
The Chamber technique, also known as the "lead-chamber process," is an industrial method for manufacturing sulfuric acid by oxidizing sulfur dioxide with moist air and employing gaseous nitrogen oxides as catalysts. The reaction is generally carried out in several sizable, box-like chambers made of sheet lead.
Simply replacing the watch glass with aluminum foil and getting the developing chamber ready before adding the TLC plates gives the solvent time to saturate, producing quicker and more repeatable results.
Therefore, the TLC plates gives the solvent time to saturate, producing quicker and more repeatable results.
Learn more about TLC plate from the given link.
https://brainly.com/question/17562109
#SPJ4
The balanced chemical equation for the reaction of hydrogen and oxygen is shown below. 2H2 + O2 Right arrow. 2H2O The number of moles of hydrogen that is needed to produce 0.253 mol of water is
Answers
Answer:
The number of moles of hydrogen that is needed to produce 0.253 mol of water is 0.253.
Explanation:
The balanced reaction is:
2 H₂ + O₂ → 2 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
H₂: 2 moles O₂: 1 mole H₂O: 2 molesThen you can apply the following rule of three: if by stoichiometry 2 moles of water are produced from 2 moles of hydrogen, 0.253 moles of water are produced from how many moles of hydrogen?
\(amount of moles of hydrogen=\frac{0.253 moles of water* 2moles of hydrogen}{2 moles of water}\)
amount of moles of hydrogen= 0.253 moles
The number of moles of hydrogen that is needed to produce 0.253 mol of water is 0.253.
Answer:
0.253 mol
Explanation:
Non-ferrous metal is NOT hardenable by heat treatment; it must
gain strength through a process such as tempering. Is this
statement TRUE or FALSE?
Group of answer choices
True
False
Answers
The statement is FALSE. Non-ferrous metals can be hardened by heat treatment, although the mechanisms and processes involved may differ from ferrous metals.
Heat treatment techniques such as precipitation hardening can be used to increase the strength of non-ferrous metals. Non-ferrous metals are metals or alloys that do not include iron (or iron allotropes, such as ferrite, etc.) in significant quantities. Non-ferrous metals are employed because they have desired qualities like reduced weight (for example, aluminium), greater conductivity (for example, copper), non-magnetic characteristics, or corrosion resistance (for example, zinc), even though they are often more expensive than ferrous metals. In the iron and steel sectors, several non-ferrous materials are also employed. Bauxite, for instance, is used as a flux in blast furnaces, whereas wolframite, pyrolusite, and chromite are utilised to create ferrous alloys.
To know more about Non-ferrous metals
https://brainly.com/question/33291477
#SPJ11
FAST 30 POINTS ON BRAINLY!!!

Answers
Is Mg(CH3COO)2 soluble or insoluble
Answers
Answer:
It is Soluble
Explanation:
Name- Magnesium acetate
It dissolves easily in water and alcohol.
Apt A piece of chalk becomes shorter as it is used. Which of the following is NOT true of the shorter piece of chalk?
A. Its mass has changed.
B. Its shape has changed.
C. Its volume has changed.
D. Its characteristic properties have changed.
Answers
What has a Gibbs free energy of 0?
Answers
Pure elements has Gibbs free energy equal to 0.
The Gibbs free energy is a concept in chemistry specially of thermodynamics. The maximum amount of work that can be accomplished at a constant temperature and pressure by a closed system can be calculated using the Gibbs free energy (also known as Gibbs energy; symbol: denoted as delta G). Additionally, it offers a prerequisite for any processes like chemical reactions that might take place in such circumstances.
When a system achieves equilibrium without being pushed by an input electrolytic voltage, the Gibbs energy is the thermodynamic potential that is reduced. At the equilibrium point, its derivative w.r.t. the system's reaction coordinate vanishes.
To know more about free energy, click here,
brainly.com/question/13765848
#SPJ4
What is an ion? Describe and give an example.
Answers
The reason that atomic numbers are always integer values is because every element has: Select the correct answer below: a. a particular number of neutrons b. a particular number of electrons
c. a particular number of protons d. a particular atomic mass
Answers
The reason that atomic numbers are always integer values is because every element has a particular number of protons (option c).
Atomic number is defined as the number of protons present in the nucleus of an atom. Protons are positively charged particles, and their number determines the identity of the element. Since protons cannot be split into smaller particles, the atomic number is always a whole number.
This is in contrast to atomic mass, which takes into account both protons and neutrons and can result in non-integer values due to isotopes. The number of electrons in a neutral atom is equal to the number of protons, but it is the protons that define the atomic number. Hence, c is the correct option.
You can learn more about atomic numbers at: brainly.com/question/8834373
#SPJ11
the formula for caffeine is c8h10n4o2. how many total atoms are in 0.75 moles of caffeine
Answers
In 0.75 moles of caffeine, there are a total of 6 carbon atoms, 7.5 hydrogen atoms, 3 nitrogen atoms, and 1.5 oxygen atoms.
To determine the total number of atoms in 0.75 moles of caffeine, we need to consider the molecular formula of caffeine, which is C8H10N4O2. The molecular formula provides the ratios of each element present in the compound. By multiplying the number of atoms in each element by the corresponding coefficient in the molecular formula, we can calculate the total number of atoms. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms in each molecule of caffeine. Multiplying these values by 0.75 moles will give us the total number of atoms in 0.75 moles of caffeine.
The molecular formula of caffeine, C8H10N4O2, provides the number of atoms for each element present in one molecule of caffeine. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms.
To calculate the total number of atoms in 0.75 moles of caffeine, we need to multiply the number of atoms for each element by the coefficient in the molecular formula, and then multiply that by the number of moles (0.75 moles).
For carbon (C): 8 atoms x 0.75 moles = 6 atoms (since there are 8 carbon atoms in one molecule of caffeine).
For hydrogen (H): 10 atoms x 0.75 moles = 7.5 atoms (since there are 10 hydrogen atoms in one molecule of caffeine).
For nitrogen (N): 4 atoms x 0.75 moles = 3 atoms (since there are 4 nitrogen atoms in one molecule of caffeine).
For oxygen (O): 2 atoms x 0.75 moles = 1.5 atoms (since there are 2 oxygen atoms in one molecule of caffeine).
To learn more about molecular click here:
brainly.com/question/156574
#SPJ11
if a substance x has a solubility of 2.4×10−5 mg/l, and a molar mass of 188 g/mol, what is the molar solubility of the substance?
Answers
To find the molar solubility of substance X, we need to convert the given solubility from milligrams per liter (mg/L) to moles per liter (mol/L). The molar solubility of substance X is 1.28 × 10^(-10) mol/L.
Step 1:
We need to convert 2.4×10−5 mg/L to grams per liter (g/L) by dividing by 1000, which gives us 2.4×10−8 g/L.
Solubility = 2.4 × 10^(-5) mg/L
1 mg = 0.001 g, so:
Solubility = 2.4 × 10^(-5) × 0.001 g/L = 2.4 × 10^(-8) g/L
Step 2:
Next, we need to convert the mass to moles using the molar mass of substance X.
Molar mass = 188 g/mol
Molar solubility =2.4×10−8 g/L ÷ 188 g/mol = 1.28×10−10 mol/L
This is the molar solubility of substance X.
The molar solubility of substance X is 1.28×10−10 mol/L.
To know more about solubility visit:
brainly.com/question/31493083
#SPJ11
For propanoic acid (HC3H5O2, Ka = 1.3 × 10–5), determine the concentration of all species present, the pH, and the percent dissociation of a 0.100-M solution.
Answers
The percent dissociation of the propanoic acid and the pH of the species are [HCO₃⁻] = 1.53 x 10⁻⁴M and [CO₃²⁻] = 4.8 x 10⁻¹¹ M.
Propionic acid, having the chemical formula CH₃CH₂CO₂H, is a naturally occurring carboxylic acid. It is a liquid with a strong, foul scent that resembles body odour. Propionates or propanoates are names for the propionic acid salts and esters as well as the anion CH₃CH₂CO₂⁻.
Propanoic acid: HC₂H,O₂ (or) C₂H,COOH Concentration of C,H,COOH = 0.290 M ICE table:
C₂H,COOH + H₂O
C₂H,COO+ H₂O*
Acid ionization constant, K 1.3×10= [C₂H,COO-][H₂O*] [C₂H,COOH] x² (0.290-x)
Since C₂H,COOH is a weak acid, we can assume that (0.290x) M= 0.290 M 1.3×10= 0.290 x= √(1.3×10) (0.290) = 1.94×10-3
According to the equilibrium table, [H3O+] = (x)M=1.94×103 M
[C₂H,COO-]=(x)M= 1.94×103 M [C₂H,COOH ]=(0.290-x)M = (0.290-1.94×103) M s 0.28806 M
s 0.288M
pH = -log[H₂O* ] = log(1.94×103) = 2.71 рон = 14.00-pH = 14.00-2.71 = 11.29 [OH-]= 10-10-1129 = 5.13×10-12 M
Percent dissociation = HCHO ] 1.94×10-3 0.290 M ×100% -x100% 0.669% = 0.67%.
Learn more about Propanoic acid:
https://brainly.com/question/20318658
#SPJ4
You need to prepare 100.0 mL of a pH 4.00 buffer solution using 0.100M benzoic acid (pK
a
=4.20) and 0.240M sodium benzoatc. How many milliliters of each solution should be mixed to prepare this buffer? benzoic acid:
Previous question
Answers
To prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.The ratio of benzoic acid to sodium benzoate in the buffer solution using the Henderson-Hasselbalch equation.
To prepare a pH 4.00 buffer solution using benzoic acid and sodium benzoate, we need to calculate the appropriate volumes of the 0.100 M benzoic acid and 0.240 M sodium benzoate solutions.
First, we need to determine the ratio of benzoic acid to sodium benzoate in the buffer solution. The Henderson-Hasselbalch equation can help us with this calculation:
pH = pKa + log([A-]/[HA])
Given that the pH is 4.00 and pKa is 4.20, we can rearrange the equation:
log([A-]/[HA]) = pH - pKa
log([A-]/[HA]) = 4.00 - 4.20
log([A-]/[HA]) = -0.20
Next, we take the antilog of -0.20 to find the ratio of [A-] to [HA]:
[A-]/[HA] = antilog(-0.20)
[A-]/[HA] = 0.63
The ratio of [A-] to [HA] is 0.63.
Now, let's calculate the volumes of each solution needed. Let's assume x represents the volume (in mL) of the 0.100 M benzoic acid solution and y represents the volume (in mL) of the 0.240 M sodium benzoate solution.
Since the total volume is 100.0 mL, we have the equation: x + y = 100
Considering the ratio of [A-] to [HA] as 0.63, we can write the equation: y/x = 0.63
Solving these two equations simultaneously will give us the volumes of each solution:
x + y = 100
y/x = 0.63
By substituting y = 0.63x from the second equation into the first equation, we get:
x + 0.63x = 100
1.63x = 100
x = 61.35 mL (rounded to two decimal places)
Substituting this value back into the equation x + y = 100, we find:
61.35 + y = 100
y = 38.65 mL (rounded to two decimal places)
Therefore, to prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.
To know more about the Henderson-Hasselbalch equation, click here, https://brainly.com/question/31732200
#SPJ11
what is the formula for P & Cl
Answers
Answer:
I belive it is PCl3
It's
( IUPAC NAME)
Phosphorus trichloride \( \bf \blue {\fbox{PCl_{3} }}\)
(other NAME)
Phosphorus (lll) chloride

Which of the following is NOT part of the cell theory?
[a] all living things are made of one or more cells
[b] all cells come from existing cells
[c] cells are the basic units of structure and function in living things
[d] your brain is constantly making new cells
Answers
the answer is B) All cells come from existing cells
The point which is not a part of the cell theory is your brain is constantly making new cells.
What is cell theory?Cell theory is a scientific theory and in this theory following three basic points are mentioned:
Cells are the basic and fundamental unit of all living life.New cells are formed from the cells which are already present.All living organisms are composed of cells.So, your brain is constantly making new cells is not the part of cell theory.
To know more about cell theory, visit the below link:
https://brainly.com/question/24595992
why should you never place a hot object on the pan of a balance
Answers
Answer:
Because, it would make the pan hot and if it's plastic then it would melt. if it was metal it might make the metal piece hot and you'd risk burning yourself.
Explanation:
You should never place a hot object on the pan of a balance because it can cause the balance to become inaccurate and affect the measurement results.
Balances are precision instruments used to measure the mass of objects with high accuracy. The balance pan and its components are designed to operate under specific conditions, including a stable temperature. When you place a hot object on the balance pan, several issues can arise:
Thermal Expansion: Materials, including metals and plastics, can expand when exposed to heat. Placing a hot object on the pan can cause the pan and its supporting components to expand, potentially affecting the balance's calibration and accuracy.
Uneven Heating: If the hot object is not uniformly heated, it can cause uneven expansion of the balance pan. This can lead to imbalanced weight distribution and inaccurate measurements.
Calibration Shift: The heat from the hot object can cause the materials in the balance to change temperature rapidly, potentially affecting the calibration of the instrument. The balance may require time to stabilize before accurate measurements can be obtained.
Damage to the Balance: Exposing the balance pan to extreme heat can cause warping, cracking, or other structural damage to the pan material. This can permanently impair the balance's performance and accuracy.
To ensure accurate measurements and maintain the integrity of the balance, it's important to allow hot objects to cool to room temperature before placing them on the balance pan. Additionally, using appropriate containers or holders designed for high-temperature applications can help prevent damage to both the hot object and the balance equipment.
To learn more about Weighing balance, here
https://brainly.com/question/33716206
#SPJ3
Forming glycogen as energy storage in the liver is an example of ________.
Answers
Forming glycogen as energy storage in the liver is an example of anabolism.
4.90 ll of a 0.175 m cacl2m cacl2 solution xpress your answer with the appropriate units. Value g Submit Previous Answers Request Answer X Incorrect; Try Again; 9 attempts remaining Part C 225 mL of a 2.50 M NaNO3 solution Express your answer with the appropriate units. Value g Submit Previous Answers Request Answer
Answers
Amount of calcium chloride ( \(CaCl_{2}\) ) there are 0.8575 moles of \(CaCl_2\) in 4.90 L of 0.175 M \(CaCl_2\)solution. Amount of \(NaNO_3\) there are 0.5625 moles of \(NaNO_3\) in 225 mL of 2.50 M \(NaNO_3\)solution.
For the first part:
Given, volume of \(CaCl_{2}\) solution = 4.90 L
Concentration of \(CaCl_{2}\) solution = 0.175 M
To find the amount of calcium chloride (\(CaCl_{2}\)) in the solution, we need to multiply the volume of the solution with the concentration of the solution. The unit of the answer will be moles (mol).
Amount of \(CaCl_{2}\) = Volume of solution × Concentration of solution
Amount of \(CaCl_{2}\) = 4.90 L × 0.175 mol/L
Amount of \(CaCl_{2}\) = 0.8575 mol
Therefore, there are 0.8575 moles of \(CaCl_{2}\) in 4.90 L of 0.175 M \(CaCl_{2}\) solution.
For the second part:
Given, volume of \(NaNO_3\) solution = 225 mL = 0.225 L
Concentration of \(NaNO_3\) solution = 2.50 M
To find the amount of Sodium nitrate (\(NaNO_3\)) in the solution, we need to multiply the volume of the solution with the concentration of the solution. The unit of the answer will be moles (mol).
Amount of \(NaNO_3\) = Volume of solution × Concentration of solution
Amount of \(NaNO_3\) = 0.225 L × 2.50 mol/L
Amount of \(NaNO_3\) = 0.5625 mol
Therefore, there are 0.5625 moles of \(NaNO_3\) in 225 mL of 2.50 M \(NaNO_3\) solution.
Learn more about calcium chloride : brainly.com/question/22284620
#SPJ11
True or false? The two types of nucleic acids found in living organisms are ribose and deoxyribose
Answers
False. The two types of nucleic acids found in living organisms are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). Ribose and deoxyribose are not nucleic acids; instead, they are sugar molecules that form the backbone of these nucleic acids.
In DNA, the sugar molecule is deoxyribose, while in RNA, it is ribose. Nucleic acids are composed of repeating units called nucleotides, which consist of a sugar molecule (ribose or deoxyribose), a phosphate group, and a nitrogenous base. DNA and RNA play essential roles in the storage and expression of genetic information within an organism, with DNA being the main genetic material and RNA serving as an intermediate in the production of proteins.
For more information on Nucleic acids see:
https://brainly.com/question/11309892
#SPJ11
What is the percent sodium in sodium chloride?
Answers
The total mass of sodium chloride is 58.44 g/mol.
The mass of sodium is 22.99 g/mol.
To find the percent sodium in sodium chloride can be found by dividing the amounts.
\(\frac{22.99}{58.44}\approx0.39\)Therefore, the percent sodium is 39%.
What is the difference between a mixture and a pure substance?
Answers
Answer:
a pure substance consists only of one element or one compound. a mixture consists of two or more different substances, not chemically joined together.
Explanation:
Answer:
Two substances mixed to get another new substance is called mixture
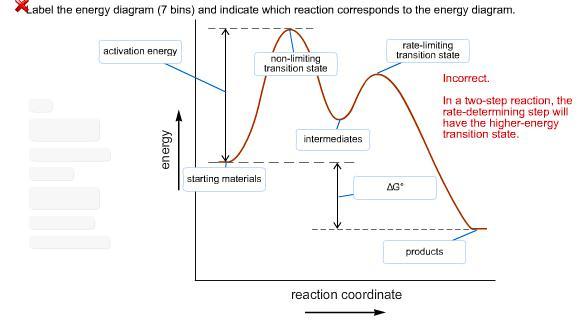
label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram.
Answers
The energy diagram, consisting of seven bins, will be labeled, and the corresponding reaction will be identified.
An energy diagram represents the energy changes that occur during a chemical reaction. In this case, the energy diagram will consist of seven bins, which represent different energy levels or states of the reactants and products.
To label the energy diagram, each bin will be assigned a corresponding energy value. The reactants will be placed in a specific bin, indicating their initial energy level.
The energy barrier or transition state will be identified as the highest point on the energy diagram, separating the reactants from the products. The products will be placed in another bin, indicating their final energy level.
Once the energy diagram is labeled, the corresponding reaction can be identified by considering the changes in energy during the reaction. The reactants will have a higher energy than the products, and the energy barrier represents the activation energy required for the reaction to proceed.
By examining the energy changes and transitions depicted on the energy diagram, it becomes possible to determine which specific reaction the diagram corresponds to. The energy diagram provides a visual representation of the energy profile of the reaction, aiding in the understanding of the reaction's thermodynamics and kinetics.
Learn more about corresponding reaction here:
https://brainly.com/question/28892861
#SPJ11
3. Differentiate between saturated and unsaturated fats
Answers
Answer:
Fats that are tightly packed with no double bonds between the fatty acids are called saturated fats.
Explanation:
pH of a 0.00072 M HCl solution.
Answers
Answer:
3.14
Explanation:
pH=-log(H+)
- Hope that helps! Please let me know if you need further explanation.
what is 2.5 meters= to mm
Answers
Answer:
2500 mm
Explanation:
2.5 m = 2.5 * 1000 = 2500 mm
Answer:
2500 mm
Explanation:
1 metre = 1000mm
Now,
2.5 metre = 2.5*1000 mm
= 2500 mm
Which statement is true of gamma radiation?
A. Gamma radiation can pass through your body
B. Gamma radiation is always dangerous to the body.
C. Gamma radiation is produced when a neutron turns into a preton
D. Gamma radiation cannot go through surfaces rather, it will beures
off them.
Answers
Answer: A. Gamma radiation can pass through your body
Explanation:
Let's use the Process of Elimination for this problem.
We will start off with B. Gamma radiation is always dangerous to the body.
Facts: Gamma radiation are not always dangerous to the body. Most of the time, Gamma rays and radiation will go through with little to no effect at all. So, now we know that the answer can not be B. Gamma radiation is always dangerous to the body.
Next, let's check if C. Gamma radiation is produced when a neutron turns into a proton is true.
Facts: Gamma radiation has nothing to do with neutrons turning into protons and protons turning into neutrons. Gamma rays are a type of radiation that is everywhere. Gamma rays do not make neutrons into protons. Now, we will eliminate option C. Gamma radiation is produced when a neutron turns into a proton.
Let's check if D. Gamma radiation cannot go through surfaces rather, it will beures off them is true or not is true or not.
Facts: Gamma rays can enter your body, they do not just bounce off of your body. Gamma rays are invisible and have no weight. They are like lasers and they can enter your body. Now, we know the answer should not be D. Gamma radiation cannot go through surfaces rather, it will beures off them is not a true statement.
Finally, let's check if A. Gamma radiation can pass through your body is true or not.
Facts: Gamma rays can pass through your body because it is like a natural laser. They can also cause ionizations that can damage tissue in your body. But most of the time, the damage to your tissue is minor. So, we know A. Gamma radiation can pass through your body is a true statement.
Therefore, we got our answer. A. Gamma radiation can pass through your body is the correct option.
ice added to a hot soup for the purpose must be made from what type of water
Answers
When adding ice to a hot soup, it is generally recommended to use ice made from potable or drinkable water.
The water used to make the ice should be clean and free from any contaminants that could affect the taste or safety of the soup.
It is advisable to use filtered or purify water to make the ice to ensure that it is of good quality. This helps prevent any unwanted flavors or impurities from transferring to the soup.
Using tap water can also be acceptable if it meets the drinking water standards in your area and is considered safe for consumption. However, if you have concerns about the quality of your tap water, using filtered water is a safer option.
Ultimately, the goal is to add ice made from water that is safe and of good quality to avoid any negative impact on the taste or safety of the soup.
To know more about purify water here
https://brainly.com/question/13704419
#SPJ4
27. Interpreting Concepts One way to make lemonade is
to start by combining lemon juice and water. To make
the lemonade taste better you could add some sugar.
Is your lemonade-sugar combination classified as a
compound or a mixture? Explain your answer.
Answers
Explanation:
compound is already together use your brain man you take multiple things. to make lemonade aka a mixture of powder and water cmon man u gotta think about it logically
think about like this a bartebder asjs you what drink your having what is he going to do next ? hes going to make the drink so you can have another right? yha
observe what happens next
the bartender grabs a cup and goes to the ice and scoops it up he takes a bottle of vodka and puts it into the shot cup "a preset cup for alcohol measurement " he takes stawberry puraee (strawberry mix) and pyrs it into the drink and mixes it around by shaking it . puts a extractor on top of it and takes a glass and pours the drink in front of you into the glass. you dont see the vodka in there do you ? no you dont because its mixed in there same thing with lemonade.
Iron (III) oxide is formed when Iron combines with oxygen in the air. How many grams of Fe₂O₃ are formed when 16.7 grams of reacts completely with oxygen? 4Fe + O₃ ---> 2Fe₂O₃
Answers
Answer:
23.9g of Fe₂O₃ are produced
Explanation:
Are formed when 16.7g of Fe reacts completely...
Based on the reaction:
4Fe + O₃ → 2Fe₂O₃
4 moles of Iron react per 1 mole of O₃ producing 2 moles of Fe₂O₃.
To solve this question we need to convert the mass of iron to moles. The ratio of reaction is 2:1 -That is, 2 moles of Fe produce 1 mole of Fe₂O₃-. Thus, we can find the moles of Fe₂O₃ produced and its mass:
Moles Fe -Molar mass: 55.845g/mol-:
16.7g Fe * (1mol / 55.845g) = 0.299 moles of Fe
Moles Fe₂O₃:
0.299 moles Fe * (2 mol Fe₂O₃ / 4 mol Fe) = 0.150 moles Fe₂O₃
Mass Fe₂O₃ -Molar mass 159.69g/mol-:
0.150 moles Fe₂O₃ * (159.69g / mol) =
23.9g of Fe₂O₃ are produced
¿ que uso e damos ala tecnología en nuestra vida diaria ? ¿que impacto tendría el no contar con la tecnología? ¿cuales son las nuevas tecnologías? ayúdenme por favor
Answers
Answer:
We in daily life use technology to pay virtually through virtual wallets, work at home, purchase packages to travel abroad, many businesses handle virtual advertising, that is why it is called the new "market window" to social networks or internet pages, university universities playful methods through virtual campuses or virtual classes.
The serious impact is very great since many of the activities of daily life change, and they will return to being as before the technology affected in our lives, reversing customs and technological generations.
The new technologies are: computer science, engineering, arquetectonica, scientific, industrial, commercial ... Where a fourth industrial revolution arises where many processes that were carried out by humans today would be automated by computers or technological devices that improve market efficiency, of consumption and industrialization.
Explanation:
In addition, new technologies are those that are based on electronic devices that are based on social communication, the development of social networks and technology based on global communications.