Answers
The result in binary would be 10000011110101100000.
By constantly dividing the supplied number by 2 until we receive 0 as the quotient, we can convert a decimal number to a binary number in the simplest way possible. The binary value of the specified decimal number is then obtained by writing the remainders in reverse order. A binary number is made up of two 0s and 1s. Two is used as the base representation for binary numerals. As an illustration, (101)2 (101) 2. Switches can be used to encode and store numbers in binary form. This system is compatible with a variety of digital devices, including computers, calculators, digital TV decoder boxes, cell phones, burglar alarms, watches, and more. Binary values are kept in memory, which is essentially a collection of electronic on/off switches.
The complete question is- Convert 540000 to Binary form.
Learn more about binary here-
https://brainly.com/question/19802955
#SPJ9
Related Questions
If I Gave A Pig Bacon Would He Eat It ?
Answers
Consider the neutralization reaction
2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq)
A 0.105 L sample of an unknown HNO3 solution required 41.1 mL
of 0.150 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3 solution?
Concentration:
Answers
The concentration of the HNO3 solution is 0.117 M.
To solve this problem, we can use the balanced chemical equation to determine the mole ratio of HNO3 to Ba(OH)2:
2HNO3(aq) + Ba(OH)2(aq) ⟶ 2H2O(l) + Ba(NO3)2(aq)
From the equation, we see that 2 moles of HNO3 react with 1 mole of Ba(OH)2 to produce 2 moles of H2O and 1 mole of Ba(NO3)2. Therefore, the moles of HNO3 in the unknown solution can be calculated from the volume and concentration of Ba(OH)2 used:
moles of Ba(OH)2 = concentration × volume = 0.150 M × 0.0411 L = 0.006165 mo
moles of HNO3 = 2 × moles of Ba(OH)2 = 2 × 0.006165 mol = 0.01233 mol
Finally, we can calculate the concentration of the HNO3 solution:
concentration = moles / volume = 0.01233 mol / 0.105 L = 0.117 M
For more question on concentration click on
https://brainly.com/question/26255204
#SPJ11
Balance C(2)H(4)+0(2)----> CO(2)+H(2)O
Answers
Change 32°C to degree °F
Answers
Answer:
89.6 F
Explanation:
Brain-List?
Solve the problem into a decimal number

Answers
Answer:0.00004
its that because like it is
Hydrogen iodide decomposes according to the equation: 2HI(g) H 2(g) + I 2(g), K c = 0.0156 at 400ºC A 0.660 mol sample of HI was injected into a 2.00 L reaction vessel held at 400ºC. Calculate the concentration of HI at equilibrium.
Answers
Answer:
[HI] = 0.264M
Explanation:
Based on the equilibrium:
2HI(g) ⇄ H₂(g) + I₂(g)
It is possible to define Kc of the reaction as the ratio between concentration of products and reactants using coefficients of each compound, thus:
Kc = 0.0156 = [H₂] [I₂] / [HI]²
As initial concentration of HI is 0.660mol / 2.00L = 0.330M, the equlibrium concentrations will be:
[HI] = 0.330M - 2X
[H₂] = X
[I₂] = X
Where X is reaction coefficient.
Replacing in Kc:
0.0156 = [X] [X] / [0.330M - 2X]²
0.0156 = X² / [0.1089 - 1.32X + 4X² ]
0.00169884 - 0.020592 X + 0.0624 X² = X²
0.00169884 - 0.020592 X - 0.9376 X² = 0
Solving for X:
X = - 0.055 → False solution, there is no negative concentrations
X = 0.0330 → Right solution.
Replacing in HI formula:
[HI] = 0.330M - 2×0.033M
[HI] = 0.264MA sample of 23.3 g of a candy bar was burned in a calorimeter. The calorimeter was calibrated to have a heat capacity of 8.72 kcal/ °C. The heat released caused the temperature of the calorimeter to increase 15.5 °C.
Calculate the food caloric content of the candy bar in nutritional calories per gram to three significant figures. Recall that 1 nutritional calorie (Cal) = 1 kcal.
Answers
The food caloric content of the candy bar in nutritional calories per gram is 135 Cal.
Calorimeter is used to measure the amount of heat energy (Q) produced during a certain reaction. It depends on the mass of the substance (m), heat capacity (c) and the change in temperature (ΔT) during the process.
Mathematically it could be represented as,
\(\rm Q\ =\ m\times c\times \Delta\ T\)
\(\rm =\ 23.3\times 8.72\times 15.5\)
\(\rm = 3149.228\ kcal\)
The heat released during the process is 3149.228 kcal.
To calculate the nutritional calories per gram, divide the heat released by the mass in grams.
\(\rm Calories\ per\ gram\ = \frac{3149.228}{23.3}\)
\(\rm = 135.16\ kcal\)
\(\rm = 135\ Cal\)
Therefore, The food caloric content of the candy bar in nutritional calories per gram is 135 Cal.
To know more on Calorimeter, click here
https://brainly.com/question/18027686
#SPJ1
What do these two changes have in common? salt and vinegar removing tarnish from a penny mixing glue and laundry powder to create putty
Answers
A chemical reaction is started by adding the borax solution to the glue mixture. A elastic, springy new material is produced when the glue and borax molecules interact.
What use does borax solution serve?Although cleaning is borax's most well-known application, the substance is also included in a wide range of home goods, such as specialty toothpastes and mouthwashes. goods for treating acne, including lotions, skin creams, moisturizers, and sunscreen. Ceramic glaze and paint.
Are baking soda and borax interchangeable terms?Borax (sodium tetraborate) is different from baking soda (sodium bicarbonate). Both salts and widely used as "green" home cleaners, baking soda and borax have pH values of 8 and 9.5, respectively.
To know more about borax solution visit:
https://brainly.com/question/29256010
#SPJ1
which of the following are examples of colligative properties. freezing point elevation increases vapor pressure increases osmotic pressure boiling point elevation
Answers
The correct option is C Osmotic pressure
Only Osmotic pressure is a colligative property.
What is Osmotic pressure?
Osmosis is the process by which water moves from a region with a low concentration of solute to one with a greater concentration. Atoms, ions, or molecules dissolved in a liquid are known as solutes. The total amount of particles dissolved in the fluid determines the rate of osmosis. The rate of osmosis increases with the number of particles that dissolve.
Water will go to the region with the highest solute concentration if a membrane is present. The pressure produced by water across a membrane as a result of osmosis is known as osmotic pressure. The osmotic pressure rises in direct proportion to the amount of water crossing the membrane.
Learn more about Osmotic pressure from given link
https://brainly.com/question/25904085
#SPJ4
Chemistry problems
1. 1.5 moles of potassium sulfate (K SO4) were dissolved in 1000 grams of water (H2O). Find the % and Cm.
2. 10 grams of sulfuric acid (H2SO4) was added to 500 ml of 10% solution of potassium hydroxide (KOH) with a density of 1.1 g/ml. Find the mass of potassium sulfate (K SO4) formed.
3. Find the mass of the salt formed by the reaction of 7.3 grams of hydrochloric acid (HCl) with 5.6 liters (5600 ml) of ammonia (NH3).
4. Find the volume of hydrogen gas (H2) produced by the reaction of 13 grams of zinc with a solution containing 30 grams of sulfuric acid (H2SO4).
5. How much of the concentrated original solution (70%) of acetic acid is needed to prepare 500 grams of 3% (percentage solution)?
Answers
1. The % concentration is 20.7% and the molar concentration, Cm, is 1.5 M.
2. 7.8 grams of potassium sulfate will be formed.
3. 10.7 grams of ammonium chloride will be formed.
4. The volume of hydrogen gas that will be produced is 3.86 liters.
5. 21.43 grams of the 70% acetic acid is needed to prepare 500 grams of 3% acetic acid solution.
What is the percentage concentration?1. Mass of potassium sulfate = 1.5 moles * (174.26 g/mol) = 261.39 g
Mass of water (H₂O) = 1000 g
% = (mass of solute/mass of solution) x 100
% = (261.39 g / (261.39 g + 1000 g)) x 100
% ≈ 20.7%
Cm = moles of solute / volume of solution
Moles of potassium sulfate (K2SO4) = 1.5 moles
Volume of water (H2O) = 1000 g / (density of water) = 1000 g / 1 g/mL = 1000 mL = 1 L
Cm = 1.5 moles / 1 L
Cm = 1.5 M
2. The balanced equation for the reaction is:
H₂SO₄ + 2 KOH → K₂SO₄ + 2 H₂O
Molar mass of sulfuric acid (H₂SO₄) = 98.09 g/mol
Moles of sulfuric acid = 10 g / 98.09 g/mol
Moles of sulfuric acid = 0.102 mol
Based on the mole ratio of the reaction, 0.102 moles of sulfuric acid will react to form 0.102 moles of potassium sulfate.
Molar mass of potassium sulfate = 174.26 g/mol
Mass of potassium sulfate = 0.102 mol x 174.26 g/mol
Mass of potassium sulfate ≈ 17.8 g
3. The balanced equation for the reaction is:
HCl + NH₃ → NH₄ClMolar mass of hydrochloric acid (HCl) = 36.46 g/mol
Moles of hydrochloric acid (HCl) = 7.3 g / 36.46 g/mol
Moles of hydrochloric acid ≈ 0.2 mol
Based on the mole ratio of the reaction, 0.2 moles of hydrochloric acid will react to form 0.2 moles of ammonium chloride.
Molar mass of ammonium chloride (NH₄Cl) = 53.49 g/mol
Mass of ammonium chloride = 0.2 mol x 53.49 g/mol
Mass of ammonium chloride ≈ 10.7 g
4. The balanced equation for the reaction is:
Zn + H₂SO₄ → ZnSO₄ + H₂Molar mass of zinc (Zn) = 65.38 g/mol
Moles of zinc = 13 g / 65.38 g/mol
Moles of zinc ≈ 0.199 mol
Based on the mole ratio of the reaction, 0.199 moles of zinc will react to produce 0.199 moles of hydrogen gas.
Volume of sulfuric acid = 30 g / (density of H₂SO₄ )
The density of H₂SO₄ is 1.84 g/mL
Volume of sulfuric acid = 30 g / 1.84 g/mL
Volume of sulfuric acid ≈ 16.3 mL or 0.0163 L
Using the ideal gas law, the volume of hydrogen gas produced will be:
V = nRT / P
V = (0.199 mol)(0.0821 L·atm/(mol·K))(273 K) / (1 atm)
V ≈ 3.86 L
5. Assuming that the concentrated original solution of acetic acid is 100% acetic acid (CH₃COOH).
Mass of acetic acid = 500 g x (3/100) = 15 g
The concentrated original solution, however, is 70% acetic acid.
70% acetic acid (mass) = 100% acetic acid (unknown mass)
0.7 * (unknown mass) = 15 g
Solving for the unknown mass:
unknown mass = 15 g / 0.7
unknown mass ≈ 21.43 g
Learn more about percentage concentration at: https://brainly.com/question/18761928
#SPJ1
a 100um thick layer of gold is plated on a medallion that is 4.00cm in diameter and 2.00mm thick. What is the volume of gold plated in cm^3?
Answers
The volume of the gold plates in cm₃ is 2.513274 cm₃
What is volume?Volume is the space occupied by a three-dimensional object“”
Volume = {mass} {density}
Given that the thickness or height of medallion, h = 2 mm = 0.2 cm
Diameter of the medallion, d = 4 cm
Radius of medallion, r = d/2 = 2 cm⁻³
The thickness of the gold plating, x = 1 μm = 10⁻⁴ cm
The density of gold = 19.3 g cm
Now we know that the shape of a medallion is cylindrical.
Hence, the volume of the medallion before platting,
Vbefore = π.r2.h = π.(2)2.0.2 = 2.513274 cm₃
Therefore, the volume of the gold-plated is 2.513274 cm₃.
To learn more about volume, refer to the link:
https://brainly.com/question/13338592
#SPJ1
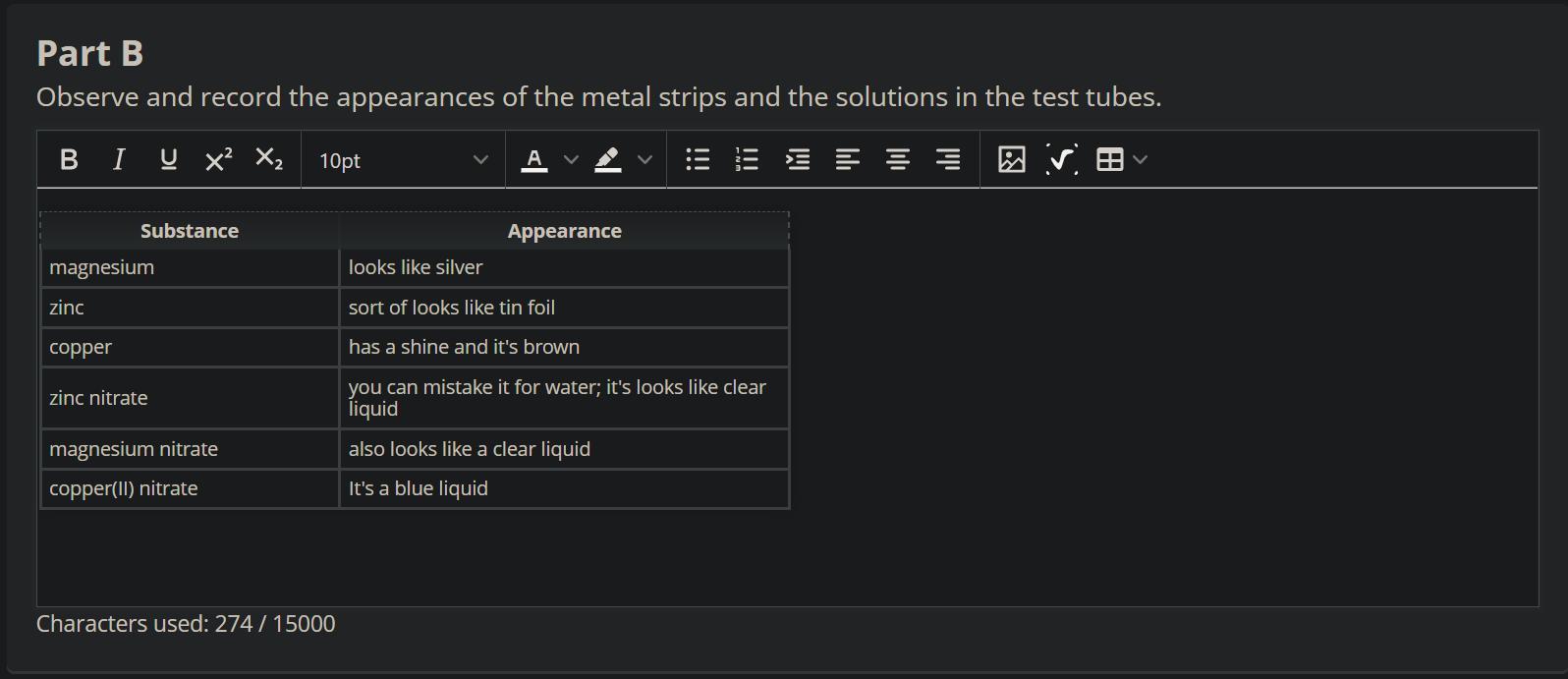
measure and record the masses of all metal strips you set out in front of the test tubes
( magnesium nitrate) ( zinc nitrate) ( copper nitrate) (magnesium ribbon) ( zinc strip ) (copper strip )
observe and record the appearances of the metal strips and the solutions in the test tubes

Answers
Magnesium ribbon dissolves in zinc nitrate and copper nitrate solutions.
Even though the question is incomplete and refers to your practical work, however, I will try to help you as much as I can.
Metals dissolves in solutions of other metals that are lower than them in the electrochemical series. Hence, copper strip will show no change in magnesium nitrate or zinc nitrate solution. A zinc strip will not show any change in magnesium nitrate or zinc nitrate.
However, a magnesium ribbon will dissolve very quickly in zinc nitrate and copper nitrate solutions.
Learn more: https://brainly.com/question/14396802
Look at the screenshot attached
1.(03.02 LC)
Which of the following best defines weather? (3 points)
Answers
The atmospheric conditions at a particular time.
i did the test.
Answer:
the atmospheric conditions at a particulur time
Explanation:
Which molecule has weakest bond?
Answers
Answer:
ionic bonds are the weakest bonds
Explanation:
Which of the following cell organelles is NOT found in BOTH plant and animal cells?
O cells membrane
O cell wall
O ribosomes
O cytoplasm
Answers
When 2.5 mol of O2 are consumed in this reaction how many moles of CO2 can be produced?
Answers
According to the reaction 8 mols of O2 produce 5 mols of CO2. Then we just need to write and use that as a conversion factor:
\(2.5molsofO_2\times\frac{5molsofCO_2}{8molsofO_2}=1.5625molsofCO_2\text{ }\)What is the energy of a wave if the frequency is 300. Hz
Answers
Answer:
\(E=1.98\times 10^{-31}\ J\)
Explanation:
Given that,
The frequency of a wave, f = 300 Hz
We need to find the energy of a wave. The formula for the energy of a wave is given by :
E = hf,
Where h is Planck's constant
\(E=6.63\times 10^{-34}\times 300\\\\=1.98\times 10^{-31}\ J\)
So, the energy of the wave is \(1.98\times 10^{-31}\ J\).
When the seed is fertilized, the sprout turns into an adult before a seedling?
Answers
3. What kind of mixture does each mixture have?
Answers
Answer:
heterogeneous and homogeneous
Explanation:
la po ba abc?
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. In essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. Suppose a current of 0.270 A is passed through an electroplating cell with an aqueous solution of Ag_2 SO_4 in the cathode compartment for 72.0 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
Mass of the pure silver deposited on a metal object made into the cathode of the cell is calculated to be 0.0217 gm.
What is electroplating?The process of using electrodeposition to coat an object in a layer of metal is called electroplating .
As we know that, Q = I * t
=0.270 * 72
= 19.44 C
Here Q is quantity of electricity , I is current in amperes = 0.270 A (given)
t is time in seconds (72.0 sec)
As 96500 Coulomb of electricity electrolyzes 1 mole of Ag
then,19.44 C of electricity deposits,
=1/96500 * 19.44
= 0.000201 moles of Ag
Mass of Ag is = number of moles * molar mass
= 0.000201 * 108
= 0.0217 gm
Thus, mass of pure silver deposited on a metal object made into the cathode of the cell is 0.0217 gm.
To know more about electroplating, refer
https://brainly.com/question/16266707
#SPJ4
how many electrons inter in to the 3d sub-shell of an atom whose atomic number is 22
Answers
The number of electrons that enter the 3d subshell of an atom whose atomic number is 22 is 2.
Why are there two electrons in the 3d subshell ?An atom with an atomic number of 22 is Titanium. It is a strong, lightweight metal that is resistant to corrosion and is used in a variety of applications, including aircraft, spacecraft, medical implants, and jewelry.
The 3d subshell can hold up to 10 electrons, but Titanium only has 2 electrons in the 3d subshell. This is because the 4s subshell is lower in energy than the 3d subshell, so the electrons are filled in the 4s subshell before they are filled in the 3d subshell.
Find out more on Titanium at https://brainly.com/question/20525872
#SPJ1
Volume (V) can be measured in ______or____.O grams (g) or liters (l)O milliliters (ml) or centimeters cubed (cm3)O milliliters (ml) or grams (g)O kilograms (kg) or milligrams (mg)
Answers
According to this question, we must know that everything related to grams, means a mass of something.
So, we cannot use grams (g) or kilograms (kg) as a unit of volume.
For volume, we use liters (L), milliliters (mL), and cm^3 as we see on the written question. Also, we have plenty of units for volume in different systems used to measure.
Answer: milliliters (mL) or centimeters cubic (cm^3)
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
Does anyone know Chemistry
Answers
Answer:
so so
Explanation:
this your question?? <_>
When 161.0 mL of water at 26.0°C is mixed with 41.0 mL of water at 85.0°C, what is the final temperature
Answers
Answer: The final temperature is \(38.0^0C\)
Explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
\(heat_{released}=heat_{absorbed}\)
\(Q=m\times c\times \Delta T=m\times c\times (T_{final}-T_{initial})\)
\(-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]\)
\(-[m_1\times (T_{final}-T_1)]=[m_2\times (T_{final}-T_2)]\) (as \(c_1=c_2\))
Q = heat absorbed or released
\(m_1\) = mass of water at \(85.0^0C\) = \(volume\times density=41.0ml\times 1g/ml=41.0g\)
\(m_2\) = mass of water at \(26.0^0C\) = \(volume\times density=161.0ml\times 1g/ml=161.0g\)
\(T_{final}\) = final temperature = ?
\(T_1\) = temperature of 41.0 ml of water = \(85.0^0C\)
\(T_2\) = temperature of 161.0 ml of water = \(26.0^0C\)
Now put all the given values, we get
\(-[41.0\times (T_f-85.0)^0C]=161.0\times (T_f-26.0)^0C\)
\(T_f=38.0^0C\)
Thus the final temperature is \(38.0^0C\)
In x-ray studies of crystalline peptides, Linus Pauling and Robert Corey found that the C—N bond in the peptide link is intermediate in length (1.32 Å) between a typical C—N single bond (1.49 Å) and a C=N double bond (1.27 Å). They also found that the peptide bond is planar (all four atoms attached to the C—N group are located in the same plane) and that the two α-carbon atoms attached to the C—N are always trans to each other (on opposite sides of the peptide bond).
Required:
a. What does the length of the C—N bond in the peptide linkage indicate about its strength and its bond order (i.e., whether it is single, double, or triple)?
b. What do the observations of Pauling and Corey tell us about the ease of rotation about the C—N peptide bond?
Answers
Answer:
The C-N bond is a double bond.
There is no free rotation about the C-N bond
Explanation:
Linus Pauling and Robert Corey carried out a painstaking study of the bond lengths of peptide bonds as well as the stereo chemistry of atoms and groups around the peptide bonds in the crystal structures of molecules containing one or a few peptide bonds.
Their findings indicate that the bond length of the C-N bond in crystalline peptides is intermediate in length (1.32 Å) between a typical C—N single bond (1.49 Å) and a C=N double bond (1.27 Å). This is because the peptide bond has some double bond character as a result of resonance involving the C=O and -NH moieties of the amide.
As a result of resonance as shown above, the peptide bonds are found to be planar hence free rotation about the C-N bond is hindered. The peptide bond is mostly found in the trans configuration because it is more energetically favourable than the cis configuration.
Summarily, owing to the existence of a partial double bond character between the α carbon and the amine nitrogen in the peptide, there is no free rotation around the C-N bond.
Liquid ammonia has a vapor pressure of 109 mm Hg at -66C and a heat of vaporization of 2.46 x 10^4 J/mol.
Estimate the normal boiling point of ammonia.
Answers
It is stated in this query that p above p is the rule when using the Clausius-Claparon equation.
How hot does it get when it boils?According to what I'm going to explain, p not will be our boiling point, and that value will be equal to delta minus. The boiling point is determined by the formula: H, vapor pressure over r 1 over t minus 1 over t not and again t not. Thus, a pressure line can be seen here. According to what we've been told, it is 1 o 9 miles per hour. 680 would enter there, followed by delta h, at a distance of 6080000000 meters from Mercury. The vapourization factor is 2.46. I'm not going to include the units in this section because ideal gas has a constant 8.314 joules per mole.
It is - 66 degrees Celsius when mole kelvin is subtracted from the initial temperature. Since our boiling point would be 2 o 7 kelvin minus 1 over t, that would convert to that value. This will be plugged into my equation using the solver here, which yields 237 kelvin when accounting for t. The boiling point at 680 millimeters of mercury would be minus 273, which gives me a result of minus 36 degrees celsius, and we then translated that to degrees celsius.
For more information on boiling point kindly visit to
https://brainly.com/question/2153588
#SPJ1
How many molecules of HCI would react with 1 mole of Mgo?
MgO + 2HCI — MgCl2 + H20
A. 3,01 x 1023
B. 1.204 x 1023
O c. 6.02 x 1023
O D. 1.204 x 1024
Answers
Answer: option d
Explanation: a p e x
One mole of HCl contains 6.022 × 10²³ molecules. 2 moles of HCl reacts with one mole of MgO. Thus, 1.2 × 10²⁴ molecules of HCl will reacts with one mole of MgO.
What is one mole?One mole of any compound contains 6.022 × 10²³ molecules. This number is called Avogadro number. Thus,one mole hydrochloric acid contains 6.022 × 10²³ HCl molecules. Similarly one mole of every compound is composed of 6.022 × 10²³ molecules that constitutes the compound.
One mole of hydrogen chloride contains 6.022 × 10²³ molecules.
Then, 2 moles of HCl = 2 × 6.022 × 10²³ = 1.2 × 10²⁴ molecules
These much HCl molecules would reacts with one mole of MgO
Therefore, there would be 1.2 × 10²⁴ molecules of HCl to react with one mole of MgO.
To find more on Avogadro number, refer here:
http://brainly.com/question/11907018
#SPJ5
How many equivalents of acid are in an acid sample that requires 23.67 mL of 0.1467 N
NaOH solution to reach the endpoint?
Answers
What is the percent composition of Fluorine (F) in the compound XeF6?
Od
26.258%
12.520%
110.76%
46.472%
Answers
The percent by mass of the fluorine in the compound is 46.472%.
What is the percent by mass?We know that the percent by mass has to do with the ratio of the total mass of the atom that is part of the compound and the total molar mass of the compound multiplied by one hundred.
The question in this case has demanded that we ought to obtain the mass percent of fluorine from the compound that we can be able to identify from the formula of the compound that is shown here as xenon hexa fluoride.
Mass of the compound can be obtained from; 131 + 6(19)
= 245 g/mol
The total mass of the fluorine atom in the compound is 114 g
Thus we have the use of; 114 /245 * 100/1
= 46.472%
The percent by mass is now gotten for the fluorine atom as 46.472%.
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1